Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-01-07 , DOI: 10.1016/j.bmc.2021.116001 Christian Bailly 1
|
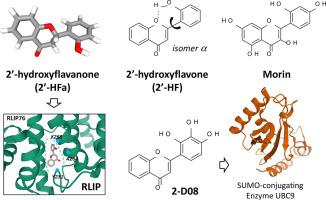
Flavonoids are abundant in nature, structurally very diversified and largely investigated. However, the subgroup of 2′-hydroxyflavonoids is much less known and not frequently studied. The present review identifies the major naturally-occurring and synthetic 2′-hydroxyflavonoid derivatives and discusses their structural characteristics and biological properties, with a focus on anticancer activities. The pharmacological properties of 2′-hydroxyflavone (2′-HF) and 2′-hydroxyflavanone (2′-HFa) are detailed. Upon binding to the Ral-interacting protein Rlip implicated in the transport of glutathione conjugates, 2′-HFa inhibits tumor cell proliferation and restrict tumor growth, in particular in breast cancer models. Among the synthetic derivatives, the characteristics of the anticancer product 2D08 (2′,3′,4′-trihydroxy flavone) are detailed to shed light on the molecular mechanism of action of this compound, as a regulator of protein SUMOylation. Inhibition of protein SUMOylation by 2D08 blocks cancer cell migration and invasion, and the compound greatly enhances the anticancer effects of conventional cytotoxic drugs like etoposide. The structural role of the 2′-hydroxyl group on the phenyl C-ring of the flavonoid is discussed, notably the capacity to engage intramolecular H-bonding interactions with the O1 atom on the B-ring of the chromone unit (or the oxygen of a 3-OH group when it is presents). The 2′-hydroxyl group of flavonoid appears as a regulator of the conformational freedom between the bicyclic A-B unit and the appended phenyl C-ring, favoring the planarity of the molecule. It is an essential group accounting for the biological properties of 2′-HF, 2′-HFa and structurally related compounds. This review shed light on 2′-hydroxyflavonoids to encourage their use and chemical development.
中文翻译:

2'-羟基类黄酮亚群:分子多样性、作用机制和抗癌特性
类黄酮在自然界中很丰富,在结构上非常多样化并且被广泛研究。然而,2'-羟基类黄酮的亚群鲜为人知,也不常被研究。本综述确定了主要的天然和合成的 2'-羟基黄酮衍生物,并讨论了它们的结构特征和生物学特性,重点是抗癌活性。详细介绍了 2'-羟基黄酮 (2'-HF) 和 2'-羟基黄烷酮 (2'-HFa) 的药理特性。在与参与谷胱甘肽结合物转运的 Ral 相互作用蛋白 Rlip 结合后,2'-HFa 会抑制肿瘤细胞增殖并限制肿瘤生长,尤其是在乳腺癌模型中。在合成衍生物中,抗癌产品2D08(2′,3′,4'-三羟基黄酮)详细阐明了该化合物作为蛋白质 SUMO 化调节剂的分子作用机制。2D08 抑制蛋白质 SUMOylation 可阻止癌细胞迁移和侵袭,该化合物大大增强了常规细胞毒性药物如依托泊苷的抗癌作用。讨论了黄酮类化合物苯基 C 环上 2'-羟基的结构作用,特别是与色酮单元 B 环上的 O1 原子(或存在时为 3-OH 基团)。类黄酮的 2'-羟基似乎是双环 AB 单元和附加的苯基 C 环之间构象自由度的调节剂,有利于分子的平面性。它是解释 2'-HF、2'-HFa 和结构相关化合物生物学特性的重要基团。这篇综述阐明了 2'-羟基黄酮类化合物,以鼓励它们的使用和化学开发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号