当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Skipped Fluorination Motifs: Synthesis of Building Blocks and Comparison of Lipophilicity Trends with Vicinal and Isolated Fluorination Motifs
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acs.joc.0c02810 Robert I Troup 1 , Benjamin Jeffries 1 , Raphael El-Bekri Saudain 1 , Eleni Georgiou 1 , Johanna Fish 1 , James S Scott 2 , Elisabetta Chiarparin 2 , Charlene Fallan 2 , Bruno Linclau 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acs.joc.0c02810 Robert I Troup 1 , Benjamin Jeffries 1 , Raphael El-Bekri Saudain 1 , Eleni Georgiou 1 , Johanna Fish 1 , James S Scott 2 , Elisabetta Chiarparin 2 , Charlene Fallan 2 , Bruno Linclau 1
Affiliation
|
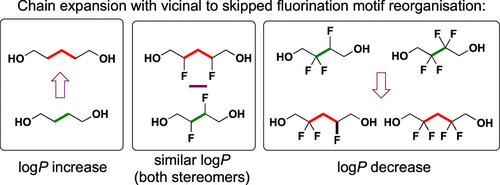
Given there is an optimal lipophilicity range for orally bioavailable drugs, structural modifications applied in the drug development process are not only focused on optimizing bioactivity but also on fine-tuning lipophilicity. Fluorine introduction can be used for both purposes. Insights into how fluorine introduction affects lipophilicity are thus of importance, and systematic series of fluorinated compounds with measured octanol–water partition coefficients are a powerful way to enhance our qualitative understanding in this regard and are essential as input for computational log P estimation programs. Here, we report a detailed comparison of all possible vicinal and skipped (1,3-substituted) fluorination motifs when embedded in structurally equivalent environments (X–CFnH2-n–CFmH2–m–X versus X–CFnH2–n–CH2–CFmH2–m–X, with n,m ≠ 0 and X = CH2OH) to compounds with isolated fluorination (n ≠ 0; m = 0, and including X–CH2–CFnH2–n–CH2–X, n = 0–2). It is shown that skipped fluorination is more powerful for log P reduction purposes compared to single or vicinal fluorination. Efficient stereoselective syntheses of the compounds with skipped fluorination motifs are reported, which where relevant can be made enantioselective using known chiral building blocks. These compounds, and some intermediates, will be of interest as advanced fluorinated building blocks.
中文翻译:

跳过的氟化基序:构建基块的合成以及亲和性和孤立氟化基序的亲脂性趋势的比较
给定口服生物利用药物的最佳亲脂性范围,在药物开发过程中应用的结构修饰不仅着眼于优化生物活性,而且着重于亲脂性的微调。氟的引入可用于两种目的。因此,对氟的引入如何影响亲脂性的见解非常重要,并且系统化的一系列具有测量的辛醇-水分配系数的氟化化合物是增强我们在这方面的定性认识的有力方法,并且是计算log P估计程序的必要输入 。在这里,我们报告了在结构等效的环境中嵌入时所有可能的邻近和跳过的(1,3-取代)氟化基序的详细比较(X– CF nH 2- n – CF m H 2– m –X与X– CF n H 2– n –CH 2 – CF m H 2– m –X,其中n,m≠0且X = CH 2 OH)具有孤立的氟化作用(n ≠0;m = 0,包括X–CH 2 – CF n H 2– n –CH 2 –X,n = 0-2)。结果表明,跳过的氟化对log P的影响更大 相比于单氟化氢或邻氟化氢的还原目的。报道了具有跳过的氟化基序的化合物的有效的立体选择性合成,可以使用已知的手性结构单元使之对映选择性。这些化合物和某些中间体将作为高级氟化构件而受到关注。
更新日期:2021-01-16
中文翻译:

跳过的氟化基序:构建基块的合成以及亲和性和孤立氟化基序的亲脂性趋势的比较
给定口服生物利用药物的最佳亲脂性范围,在药物开发过程中应用的结构修饰不仅着眼于优化生物活性,而且着重于亲脂性的微调。氟的引入可用于两种目的。因此,对氟的引入如何影响亲脂性的见解非常重要,并且系统化的一系列具有测量的辛醇-水分配系数的氟化化合物是增强我们在这方面的定性认识的有力方法,并且是计算log P估计程序的必要输入 。在这里,我们报告了在结构等效的环境中嵌入时所有可能的邻近和跳过的(1,3-取代)氟化基序的详细比较(X– CF nH 2- n – CF m H 2– m –X与X– CF n H 2– n –CH 2 – CF m H 2– m –X,其中n,m≠0且X = CH 2 OH)具有孤立的氟化作用(n ≠0;m = 0,包括X–CH 2 – CF n H 2– n –CH 2 –X,n = 0-2)。结果表明,跳过的氟化对log P的影响更大 相比于单氟化氢或邻氟化氢的还原目的。报道了具有跳过的氟化基序的化合物的有效的立体选择性合成,可以使用已知的手性结构单元使之对映选择性。这些化合物和某些中间体将作为高级氟化构件而受到关注。











































 京公网安备 11010802027423号
京公网安备 11010802027423号