当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Propane Dehydrogenation on Ga2O3-Based Catalysts: Contrasting Performance with Coordination Environment and Acidity of Surface Sites
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acscatal.0c05009 Pedro Castro-Fernández 1 , Deni Mance 2 , Chong Liu 3 , Ilia B. Moroz 2 , Paula M. Abdala 1 , Evgeny A. Pidko 3 , Christophe Copéret 2 , Alexey Fedorov 1 , Christoph R. Müller 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acscatal.0c05009 Pedro Castro-Fernández 1 , Deni Mance 2 , Chong Liu 3 , Ilia B. Moroz 2 , Paula M. Abdala 1 , Evgeny A. Pidko 3 , Christophe Copéret 2 , Alexey Fedorov 1 , Christoph R. Müller 1
Affiliation
|
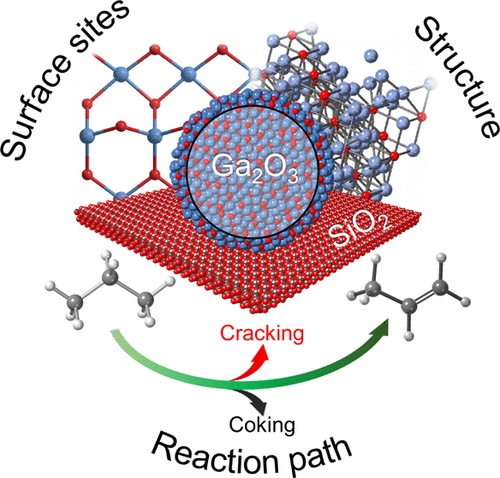
α-Ga2O3, β-Ga2O3, and γ-Ga2O3 as well as the silica-supported catalysts γ-Ga2O3/SiO2, β-Ga2O3/SiO2, and Ga(NO3)3-derived Ga/SiO2 were prepared, characterized, and evaluated for propane dehydrogenation (PDH) at 550 °C. The coordination environment and acidity of surface sites in stand-alone and SiO2-supported Ga2O3 catalysts were studied using FTIR, 15N dynamic nuclear polarization surface-enhanced NMR spectroscopy (15N DNP SENS), and DFT modeling of the adsorbed pyridine probe molecule. The spectroscopic data suggest that the Lewis acidic surface Ga sites in γ-Ga2O3 and β-Ga2O3 (the latter obtained from colloidal nanocrystals of γ-Ga2O3 via thermal treatment at 750 °C) are similar, except that β-Ga2O3 contains a larger relative fraction of weak Ga3+ Lewis acid sites. In contrast, α-Ga2O3 features mostly strong Lewis acid sites. This difference in surface sites parallels their difference in catalytic activities: i.e., weak Lewis acid surface sites are more abundant in β-Ga2O3 relative to α-Ga2O3 and γ-Ga2O3 and the increased relative abundance of weak Lewis acidity correlates with a higher initial catalytic activity in PDH, 0.41 > 0.28 > 0.14 mmol C3H6 m–2 (Ga2O3) h–1 at 550 °C, for respectively β-, α-, and γ-Ga2O3 with initial propene selectivities of 86, 83, and 88%. Dispersion of γ-Ga2O3 or β-Ga2O3 on a silica support introduces strong as well as abundant weak Brønsted acidity to the catalysts, lowering the PDH selectivity. The γ-Ga2O3/SiO2 catalyst was slightly more active than β-Ga2O3/SiO2 in PDH (Ga normalized activity) with initial propene formation rates of 11 and 9 mol C3H6 mol Ga–1 h–1 (sel = 76 and 73%, respectively). However, these catalysts deactivated by ca. 55% within 100 min time on stream (TOS) due to coking. In contrast, Ga/SiO2, with mostly tetracoordinated surface Ga sites and abundant, strong Brønsted acid sites, gave a lower activity and selectivity in PDH (3.5 mol C3H6 mol Ga–1 h–1 and 49%, respectively) but showed no deactivation with TOS. DFT calculations using a fully dehydroxylated oxygen-deficient model β-Ga2O3 surface show that tetra- and pentacoordinated Ga Lewis acid sites bind pyridine more strongly than tricoordinated Ga sites and a higher relative fraction of strong Lewis acid sites correlates with increased coking. Overall, our results indicate that weakly Lewis acidic, tricoordinated Ga3+ sites are likely driving the superior PDH activity of β-Ga2O3.
中文翻译:

Ga 2 O 3基催化剂上的丙烷脱氢:与配位环境和表面位点酸度的对比性能
α -镓2 ö 3,的β-Ga 2 ö 3,和γ -镓2 ö 3以及二氧化硅负载催化剂γ -镓2 ö 3 /的SiO 2,的β-Ga 2 ö 3 /的SiO 2,和制备,表征和表征来自Ga(NO 3)3的Ga / SiO 2,并在550℃下评价丙烷脱氢(PDH)。使用FTIR研究了独立和SiO 2负载的Ga 2 O 3催化剂中配位环境和表面位点的酸性,15N动态核极化表面增强NMR光谱(15 N DNP SENS)和吸附吡啶探针分子的DFT建模。光谱数据表明,γ-嘎路易斯酸性表面镓站点2 ö 3和的β-Ga 2 ö 3(从γ-Ga中的胶体纳米晶体获得后者2 ö 3在750℃下通过热处理)是相似的,不同之处在于的β-Ga 2 ö 3包含的弱嘎较大相对分数3+路易斯酸位点。与此相反,α-嘎2 ö 3主要具有强路易斯酸位点。在表面位点的这种差异平行它们在催化活性差:即,弱路易斯酸表面位点是在更丰富的β-Ga 2 ö 3相对于α -镓2 ö 3和γ-镓2 ö 3和增加的相对丰度弱路易斯酸度与PDH的较高初始催化活性相关,分别在550°C时分别对β-,α-和γ产生0.41> 0.28> 0.14 mmol C 3 H 6 m –2(Ga 2 O 3)h –1 -Ga 2 O 3初始丙烯选择性分别为86%,83%和88%。γ-Ga中分散2 ö 3或的β-Ga 2 ö 3在二氧化硅载体上引入了强以及丰富弱布朗斯台德酸度的催化剂,降低PDH选择性。所述γ-镓2 ö 3 /二氧化硅2催化剂是稍微比活性的β-Ga 2 ö 3 /的SiO 2在PDH(GA归一化活性)与11初始丙烯形成率和9摩尔C ^ 3 ħ 6摩尔嘎-1 h –1(sel分别为76%和73%)。然而,这些催化剂通过约10℃失活。由于焦化,在100分钟内投产(TOS)时55%。相比之下,Ga / SiO 2大部分具有四配位的表面Ga位和丰富的强布朗斯台德酸位,在PDH中的活性和选择性较低(分别为3.5 mol C 3 H 6 mol Ga –1 h –1和49%)但未显示使用TOS停用。使用完全脱羟基氧不足型的模型的β-Ga DFT计算2 ö 3表面显示,四配位和五配位的Ga路易斯酸位点比三配位的Ga位点更牢固地结合吡啶,强路易斯酸位点的较高相对分数与焦化增加相关。总的来说,我们的结果表明,弱路易斯酸性,tricoordinated嘎3+网站都可能推动的β-Ga优越的PDH活性2 Ø 3。
更新日期:2021-01-15
中文翻译:

Ga 2 O 3基催化剂上的丙烷脱氢:与配位环境和表面位点酸度的对比性能
α -镓2 ö 3,的β-Ga 2 ö 3,和γ -镓2 ö 3以及二氧化硅负载催化剂γ -镓2 ö 3 /的SiO 2,的β-Ga 2 ö 3 /的SiO 2,和制备,表征和表征来自Ga(NO 3)3的Ga / SiO 2,并在550℃下评价丙烷脱氢(PDH)。使用FTIR研究了独立和SiO 2负载的Ga 2 O 3催化剂中配位环境和表面位点的酸性,15N动态核极化表面增强NMR光谱(15 N DNP SENS)和吸附吡啶探针分子的DFT建模。光谱数据表明,γ-嘎路易斯酸性表面镓站点2 ö 3和的β-Ga 2 ö 3(从γ-Ga中的胶体纳米晶体获得后者2 ö 3在750℃下通过热处理)是相似的,不同之处在于的β-Ga 2 ö 3包含的弱嘎较大相对分数3+路易斯酸位点。与此相反,α-嘎2 ö 3主要具有强路易斯酸位点。在表面位点的这种差异平行它们在催化活性差:即,弱路易斯酸表面位点是在更丰富的β-Ga 2 ö 3相对于α -镓2 ö 3和γ-镓2 ö 3和增加的相对丰度弱路易斯酸度与PDH的较高初始催化活性相关,分别在550°C时分别对β-,α-和γ产生0.41> 0.28> 0.14 mmol C 3 H 6 m –2(Ga 2 O 3)h –1 -Ga 2 O 3初始丙烯选择性分别为86%,83%和88%。γ-Ga中分散2 ö 3或的β-Ga 2 ö 3在二氧化硅载体上引入了强以及丰富弱布朗斯台德酸度的催化剂,降低PDH选择性。所述γ-镓2 ö 3 /二氧化硅2催化剂是稍微比活性的β-Ga 2 ö 3 /的SiO 2在PDH(GA归一化活性)与11初始丙烯形成率和9摩尔C ^ 3 ħ 6摩尔嘎-1 h –1(sel分别为76%和73%)。然而,这些催化剂通过约10℃失活。由于焦化,在100分钟内投产(TOS)时55%。相比之下,Ga / SiO 2大部分具有四配位的表面Ga位和丰富的强布朗斯台德酸位,在PDH中的活性和选择性较低(分别为3.5 mol C 3 H 6 mol Ga –1 h –1和49%)但未显示使用TOS停用。使用完全脱羟基氧不足型的模型的β-Ga DFT计算2 ö 3表面显示,四配位和五配位的Ga路易斯酸位点比三配位的Ga位点更牢固地结合吡啶,强路易斯酸位点的较高相对分数与焦化增加相关。总的来说,我们的结果表明,弱路易斯酸性,tricoordinated嘎3+网站都可能推动的β-Ga优越的PDH活性2 Ø 3。































 京公网安备 11010802027423号
京公网安备 11010802027423号