European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.ejmech.2020.113136 Mathieu Gagné-Boulet , Chahrazed Bouzriba , Atziri Corin Chavez Alvarez , Sébastien Fortin
|
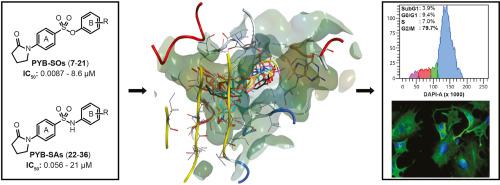
We recently designed and prepared new families of potent antimicrotubule agents designated as N-phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB–SOs) and phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonamides (PIB–SAs). Our previous structure-activity relationship studies (SAR) focused on the aromatic ring B of PIB-SOs and PIB-SAs leaving the impact of the phenylimidazolidin-2-one moiety (ring A) on the binding to the colchicine-binding site (C-BS) poorly studied. Therefore, the aim of the present study was to evaluate the effect of replacing the imidazolidin-2-one (IMZ) group by a pyrrolidin-2-one moiety. To that end, 15 new phenyl 4-(2-oxopyrrolidin-1-yl)benzenesulfonate (PYB–SO) and 15 phenyl 4-(2-oxopyrrolidin-1-yl)benzenesulfonamide (PYB-SA) derivatives were designed, prepared, chemically characterised and biologically evaluated. PYB-SOs and PYB-SAs exhibit antiproliferative activity in the low nanomolar to low micromolar range (0.0087–8.6 μM and 0.056–21 μM, respectively) on human HT-1080, HT-29, M21 and MCF7 cancer cell lines. Moreover, they block cell cycle progression in G2/M phase. Immunofluorescence, tubulin affinity and tubulin polymerisation assays show that they cause microtubule depolymerisation by docking the C-BS. In addition, docking assays with the most potent derivatives show binding affinity toward the C-BS and they also exhibit weak or no toxicity toward chick embryos. Finally, physicochemical properties calculated using the SwissADME algorithm show that PYB-SOs and PYB-SAs are promising new families of antimicrotubule agents.
中文翻译:

苯基4-(2-氧吡咯烷-1-基)苯磺酸盐和苯基4-(2-氧吡咯烷-1-基)苯磺酰胺为靶向秋水仙碱结合位点的新型抗微管剂
我们最近设计并制备了新的有效抗微管剂家族,命名为N4-(2-氧代咪唑啉-1-基)苯磺酸苯酯(PIB-SOs)和4-(2-氧代咪唑啉-1-基)苯磺酰胺(PIB-SAs)。我们之前的结构活性关系研究(SAR)专注于PIB-SO和PIB-SA的芳香环B,而苯基咪唑啉二-2-酮部分(环A)对秋水仙碱结合位点的结合(C -BS)学习得不好。因此,本研究的目的是评估由吡咯烷-2-酮部分取代咪唑烷-2--2-酮(IMZ)的效果。为此,设计,制备了15种新的4-(2-氧杂吡咯烷-1-基)苯磺酸苯基酯(PYB-SO)和15种苯基4-(2-氧杂吡咯烷-1-基)苯磺酸酰胺(PYB-SA)衍生物,化学表征和生物学评估。PYB-SO和PYB-SA在低纳摩尔至低微摩尔范围内显示抗增殖活性(0。在人HT-1080,HT-29,M21和MCF7癌细胞系上分别为0087–8.6μM和0.056–21μM)。而且,它们阻断了G2 / M期的细胞周期进程。免疫荧光,微管蛋白亲和力和微管蛋白聚合试验表明,它们通过对接C-BS引起微管解聚。另外,具有最有效衍生物的对接测定法显示出对C-BS的结合亲和力,并且它们对雏鸡胚胎的毒性也很弱或没有毒性。最后,使用SwissADME算法计算的理化性质表明,PYB-SO和PYB-SA是有希望的新型抗微管剂家族。微管蛋白亲和力和微管蛋白聚合试验表明,它们通过对接C-BS引起微管解聚。另外,具有最有效衍生物的对接测定法显示出对C-BS的结合亲和力,并且它们对雏鸡胚胎的毒性也很弱或没有毒性。最后,使用SwissADME算法计算的理化性质表明,PYB-SO和PYB-SA是有希望的新型抗微管剂家族。微管蛋白亲和力和微管蛋白聚合试验表明,它们通过对接C-BS引起微管解聚。另外,具有最有效衍生物的对接测定法显示出对C-BS的结合亲和力,并且它们对雏鸡胚胎的毒性也很弱或没有毒性。最后,使用SwissADME算法计算的理化性质表明,PYB-SO和PYB-SA是有希望的新型抗微管剂家族。






























 京公网安备 11010802027423号
京公网安备 11010802027423号