Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.colsurfa.2020.126106 Hassnain Asgar , Jiaqi Jin , Jan Miller , Ivan Kuzmenko , Greeshma Gadikota
|
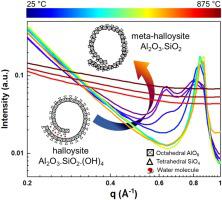
具有相似化学结构但形态组织不同的材料在热诱导化学形态演化中表现出相当大的差异。随着多尺度X射线散射测量的最新进展,现在有可能以非侵入方式探测此类材料的结构和微观结构演变。在这项研究中,我们调查了埃洛石的化学形态演变,并将结果与高岭石进行了对比。埃洛石和高岭石分别具有管状和平面形态。两种材料都是铝硅酸盐,包括以TO结构组织的二氧化硅四面体(T)和氧化铝八面体(O)片。确定了结构演化的四个不同阶段。第一,第二,第三和第四阶段对应的温度范围是25–125°C,125–400°C,400–625°C,和625–875°C。主要的结构变化对应于阶段I中层间/吸附水的去除,阶段II中不存在层间水的埃洛石结构的存在,阶段III中埃洛石的脱羟基化以及阶段IV中有序埃洛石向无定形间卤石的转化。另外,由于平均孔径从6.4 nm增加到6.6 nm,将埃洛石加热到875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 主要的结构变化对应于阶段I中层间/吸附水的去除,阶段II中不存在层间水的埃洛石结构的存在,阶段III中埃洛石的脱羟基化以及阶段IV中有序埃洛石向无定形间卤石的转化。另外,由于平均孔径从6.4 nm增加到6.6 nm,将埃洛石加热到875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 主要的结构变化对应于阶段I中层间/吸附水的去除,阶段II中不存在层间水的埃洛石结构的存在,阶段III中埃洛石的脱羟基化以及阶段IV中有序埃洛石向无定形间卤石的转化。另外,由于平均孔径从6.4 nm增加到6.6 nm,将埃洛石加热到875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 在阶段II中存在没有夹层水的埃洛石结构,在阶段III中使埃洛石脱羟基,并在阶段IV中将有序埃洛石转化为无定形间卤石。另外,由于平均孔径从6.4 nm增加到6.6 nm,将埃洛石加热到875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 在阶段II中存在没有夹层水的埃洛石结构,在阶段III中使埃洛石脱羟基,并在阶段IV中将有序埃洛石转化为无定形间卤石。另外,由于平均孔径从6.4 nm增加到6.6 nm,将埃洛石加热到875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 当平均孔半径从6.4 nm增加到6.6 nm时,将埃洛石加热至875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径 当平均孔半径从6.4 nm增加到6.6 nm时,将埃洛石加热至875°C会使纳米管略微变宽。加热还导致纳米管的壁厚从约120 nm(25°C)增加到161 nm(875°C)。埃洛石纳米管直径的增加归因于结构的扩展和表面粗糙度的增加。还使用N确认了埃洛石纳米管中的孔径2吸附-解吸和纳米X射线计算机断层扫描(nano-XCT)测量。去除层间水分后,埃洛石的层间基础间距从9.8变为7.2。在625–875°C的温度范围内,加热埃洛石会引起纳米管孔的小幅扩宽和表面积的小幅增加。相反,高岭石中的层间间距在加热时破裂,这降低了纳米级孔隙率。这些研究证明了具有管状和平面形态的硅铝酸盐在化学形态演变上的差异。

"点击查看英文标题和摘要"






























 京公网安备 11010802027423号
京公网安备 11010802027423号