Biochemical and Biophysical Research Communications ( IF 2.5 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.bbrc.2020.12.106 Wei-Chung Chiou , Jui-Chieh Chen , Yun-Ti Chen , Jinn-Moon Yang , Lih-Hwa Hwang , Yi-Shuan Lyu , Hsin-Yi Yang , Cheng Huang
|
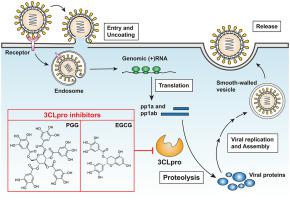
The coronavirus disease (COVID-19) pandemic, resulting from human-to-human transmission of a novel severe acute respiratory syndrome coronavirus (SARS-CoV-2), has led to a global health crisis. Given that the 3 chymotrypsin-like protease (3CLpro) of SARS-CoV-2 plays an indispensable role in viral polyprotein processing, its successful inhibition halts viral replication and thus constrains virus spread. Therefore, developing an effective SARS-CoV-2 3CLpro inhibitor to treat COVID-19 is imperative. A fluorescence resonance energy transfer (FRET)-based method was used to assess the proteolytic activity of SARS-CoV-2 3CLpro using intramolecularly quenched fluorogenic peptide substrates corresponding to the cleavage sequence of SARS-CoV-2 3CLpro. Molecular modeling with GEMDOCK was used to simulate the molecular interactions between drugs and the binding pocket of SARS-CoV-2 3CLpro. This study revealed that the Vmax of SARS-CoV-2 3CLpro was about 2-fold higher than that of SARS-CoV 3CLpro. Interestingly, the proteolytic activity of SARS-CoV-2 3CLpro is slightly more efficient than that of SARS-CoV 3CLpro. Meanwhile, natural compounds PGG and EGCG showed remarkable inhibitory activity against SARS-CoV-2 3CLpro than against SARS-CoV 3CLpro. In molecular docking, PGG and EGCG strongly interacted with the substrate binding pocket of SARS-CoV-2 3CLpro, forming hydrogen bonds with multiple residues, including the catalytic residues C145 and H41. The activities of PGG and EGCG against SARS-CoV-2 3CLpro demonstrate their inhibition of viral protease activity and highlight their therapeutic potentials for treating SARS-CoV-2 infection.
中文翻译:

PGG和EGCG对SARS-CoV-2 3C样蛋白酶的抑制作用
由新型严重急性呼吸综合征冠状病毒 (SARS-CoV-2) 人际传播引起的冠状病毒病 (COVID-19) 大流行已导致全球健康危机。鉴于 SARS-CoV-2 的 3 种糜蛋白酶样蛋白酶 (3CLpro) 在病毒多蛋白加工中发挥着不可或缺的作用,其成功抑制可阻止病毒复制,从而限制病毒传播。因此,开发一种有效的 SARS-CoV-2 3CLpro 抑制剂来治疗 COVID-19 势在必行。基于荧光共振能量转移 (FRET) 的方法使用与 SARS-CoV-2 3CLpro 的切割序列相对应的分子内猝灭荧光肽底物来评估 SARS-CoV-2 3CLpro 的蛋白水解活性。使用 GEMDOCK 进行分子建模来模拟药物与 SARS-CoV-2 3CLpro 结合袋之间的分子相互作用。本研究表明,VSARS-CoV-2 3CLpro 的最大值约为 SARS-CoV 3CLpro 的 2 倍。有趣的是,SARS-CoV-2 3CLpro 的蛋白水解活性略高于 SARS-CoV 3CLpro。同时,天然化合物 PGG 和 EGCG 对 SARS-CoV-2 3CLpro 的抑制活性比对 SARS-CoV-3CLpro 的抑制活性显着。在分子对接中,PGG 和 EGCG 与 SARS-CoV-2 3CLpro 的底物结合口袋发生强烈相互作用,与多个残基形成氢键,包括催化残基 C145 和 H41。PGG 和 EGCG 对 SARS-CoV-2 3CLpro 的活性证明了它们对病毒蛋白酶活性的抑制作用,并突出了它们治疗 SARS-CoV-2 感染的治疗潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号