当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Hydrocyanation of N‐Phosphinoyl Aldimines with Acetone Cyanohydrin by Cooperative Lewis Acid/Onium Salt/Brønsted Base Catalysis
ChemCatChem ( IF 3.8 ) Pub Date : 2021-02-02 , DOI: 10.1002/cctc.202001921 Thorsten Junge 1 , Marvin Titze 1 , Wolfgang Frey 1 , René Peters 2
ChemCatChem ( IF 3.8 ) Pub Date : 2021-02-02 , DOI: 10.1002/cctc.202001921 Thorsten Junge 1 , Marvin Titze 1 , Wolfgang Frey 1 , René Peters 2
Affiliation
|
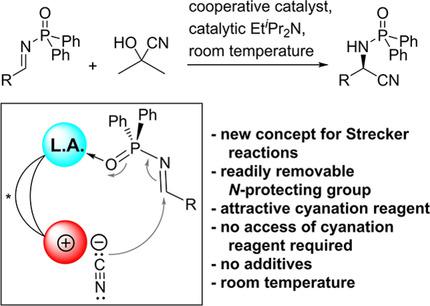
α‐Amino acids are of fundamental importance for life. Both natural and artificial α‐amino acids also play a crucial role for pharmaceutical purposes. The catalytic asymmetric Strecker reaction still provides one of the most attractive strategies to prepare scalemic α‐amino acids. Here we disclose a new concept for Strecker reactions, in which an achiral Brønsted base cooperates with a Lewis acid and an aprotic ammonium salt, which are both arranged in the same chiral catalyst entity. The described method could successfully address various long‐standing practical issues of this reaction type. The major practical advantages are that (1) the N‐protecting group is readily removable, (2) acetone cyanohydrin is attractive as cyanation reagent in terms of atom economy and cost efficiency, (3) an excess of the cyanation reagent is not necessary, (4) the new method does not require additives and (5) is performed at ambient temperature.
中文翻译:

路易斯氰/ nium盐/布朗斯台德碱催化协同作用使N-膦酰基醛亚胺与丙酮氰醇不对称氢氰化
α-氨基酸对生命至关重要。天然和人工α-氨基酸在药学上也都起着至关重要的作用。催化不对称斯特雷克反应仍然是制备规模化α-氨基酸的最有吸引力的策略之一。在这里,我们公开了Strecker反应的新概念,其中非手性布朗斯台德碱与路易斯酸和非质子性铵盐协同作用,两者都布置在相同的手性催化剂实体中。所描述的方法可以成功解决这种反应类型的各种长期存在的实际问题。主要的实际优势是(1)N-保护基易于去除;(2)从原子经济和成本效率的角度来看,丙酮氰醇作为氰化试剂具有吸引力;(3)不需要过量的氰化试剂;(4)新方法不需要添加剂;以及(5)在环境温度下进行。
更新日期:2021-03-23
中文翻译:

路易斯氰/ nium盐/布朗斯台德碱催化协同作用使N-膦酰基醛亚胺与丙酮氰醇不对称氢氰化
α-氨基酸对生命至关重要。天然和人工α-氨基酸在药学上也都起着至关重要的作用。催化不对称斯特雷克反应仍然是制备规模化α-氨基酸的最有吸引力的策略之一。在这里,我们公开了Strecker反应的新概念,其中非手性布朗斯台德碱与路易斯酸和非质子性铵盐协同作用,两者都布置在相同的手性催化剂实体中。所描述的方法可以成功解决这种反应类型的各种长期存在的实际问题。主要的实际优势是(1)N-保护基易于去除;(2)从原子经济和成本效率的角度来看,丙酮氰醇作为氰化试剂具有吸引力;(3)不需要过量的氰化试剂;(4)新方法不需要添加剂;以及(5)在环境温度下进行。













































 京公网安备 11010802027423号
京公网安备 11010802027423号