Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.jcat.2020.12.031
Zixuan Wang , Samuel D. Young , Bryan R. Goldsmith , Nirala Singh
|
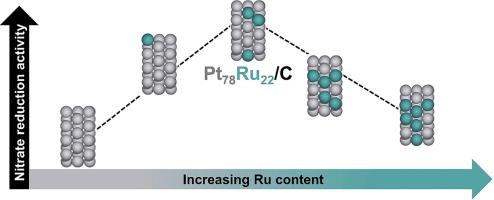
Nitrate produced from industrial and agricultural processes has imbalanced the global nitrogen cycle. Electrocatalytic reduction is a sustainable route to remediate nitrate while generating products such as ammonia or N2. Here we report the surface-area normalized activity of platinum-ruthenium (PtxRuy/C) catalysts of different compositions (x = 48–100%) for electrocatalytic nitrate reduction, chosen based on screening using a computational activity volcano plot. The PtxRuy/C alloys are more active than Pt/C, with Pt78Ru22/C six times more active than Pt/C at 0.1 V vs. RHE, and ammonia faradaic efficiencies of 93–98%. Density functional theory calculations predict maximum activity at 25 at% Ru, consistent with experiments. This maximum is due to a transition from nitrate dissociation as the rate determining step to a new rate-determining step at higher Ru content. This study demonstrates how electrocatalyst performance is tunable by changing the adsorption strength of reacting species through alloying.
中文翻译:

通过控制PtRu合金的吸附来提高电催化硝酸盐的还原活性
工业和农业生产过程中产生的硝酸盐使全球氮循环失衡。电催化还原是在产生诸如氨或N 2之类的产物时修复硝酸盐的可持续途径。在这里,我们报告了不同组成(x = 48-100%)的铂钌(Pt x Ru y / C)催化剂对电催化硝酸盐还原的表面面积归一化活性,这是根据使用计算活性火山图筛选得出的。Pt x Ru y / C合金比Pt / C更具活性,其中Pt 78 Ru 22/ C在0.1 V时的活性是Pt / C的六倍,相对于RHE,氨法拉第效率为93-98%。密度泛函理论计算预测在25 at%Ru时的最大活性,与实验一致。该最大值归因于在较高的Ru含量下从硝酸盐解离作为速率确定步骤到新的速率确定步骤的转变。这项研究表明如何通过合金化改变反应物种的吸附强度来调节电催化剂的性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号