当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective C–H Amination Catalyzed by Nickel Iminyl Complexes Supported by Anionic Bisoxazoline (BOX) Ligands
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-01-04 , DOI: 10.1021/jacs.0c09839 Yuyang Dong 1 , Colton J Lund 2 , Gerard J Porter 1 , Ryan M Clarke 1 , Shao-Liang Zheng 1 , Thomas R Cundari 2 , Theodore A Betley 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-01-04 , DOI: 10.1021/jacs.0c09839 Yuyang Dong 1 , Colton J Lund 2 , Gerard J Porter 1 , Ryan M Clarke 1 , Shao-Liang Zheng 1 , Thomas R Cundari 2 , Theodore A Betley 1
Affiliation
|
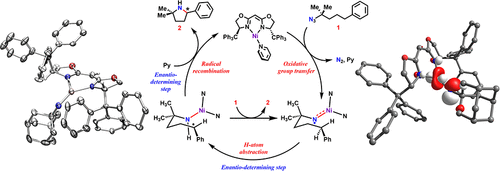
The trityl-substituted bisoxazoline (TrHBOX) was prepared as a chiral analogue to a previously reported nickel dipyrrin system capable of ring-closing amination catalysis. Ligand metalation with divalent NiI2(py)4 followed by potassium graphite reduction afforded the monovalent (TrHBOX)Ni(py) (4). Slow addition of 1.4 equiv of a benzene solution of 1-adamantylazide to 4 generated the tetrazido (TrHBOX)Ni(κ2-N4Ad2) (5) and terminal iminyl adduct (TrHBOX)Ni(NAd) (6). Investigation of 6 via single-crystal X-ray crystallography, NMR and EPR spectroscopies, and computations revealed a Ni(II)-iminyl radical formulation, similar to its dipyrrinato congener. Complex 4 exhibits enantioselective intramolecular C-H bond amination to afford N-heterocyclic products from 4-aryl-2-methyl-2-azidopentanes. Catalytic C-H amination occurs under mild conditions (5 mol % catalyst, 60 °C) and provides pyrrolidine products in decent yield (29%-87%) with moderate ee (up to 73%). Substrates with a 3,5-dialkyl substitution on the 4-aryl position maximized the observed enantioselectivity. Kinetic studies to probe the reaction mechanism were conducted using 1H and 19F NMR spectroscopies. A small, intermolecular kinetic isotope effect (1.35 ± 0.03) suggests an H-atom abstraction step with an asymmetric transition state while the reaction rate is measured to be first order in catalyst and zeroth order in substrate concentrations. Enantiospecific deuterium labeling studies show that the enantioselectivity is dictated by both the H-atom abstraction and radical recombination steps due to the comparable rate between radical rotation and C-N bond formation. Furthermore, the competing elements of the two-step reaction where H-removal from the pro-R configuration is preferred while the preferential radical capture occurs with the Si face of the carboradical likely lead to the diminished ee observed, as corroborated by theoretical calculations. Based on these enantio-determining steps, catalytic enantioselective synthesis of 2,5-bis-tertiary pyrrolidines is demonstrated with good yield (50-78%) and moderate ee (up to 79%).
中文翻译:

阴离子双恶唑啉 (BOX) 配体负载的镍亚胺基配合物催化的对映选择性 C-H 胺化
将三苯甲基取代的双恶唑啉 (TrHBOX) 制备为先前报道的能够进行闭环胺化催化的联吡啶镍系统的手性类似物。用二价 NiI2(py)4 进行配体金属化,然后还原钾石墨,得到一价 (TrHBOX)Ni(py) (4)。将 1.4 equiv 的 1-金刚基叠氮化物的苯溶液缓慢添加到 4 中生成四叠氮基 (TrHBOX)Ni(κ2-N4Ad2) (5) 和末端亚胺基加合物 (TrHBOX)Ni(NAd) (6)。通过单晶 X 射线晶体学、NMR 和 EPR 光谱学和计算对 6 进行的研究揭示了一种 Ni(II)-亚胺基自由基配方,类似于其双吡喃酮同系物。配合物 4 表现出对映选择性分子内 CH 键胺化,可从 4-aryl-2-methyl-2-azidopentanes 得到 N-杂环产物。催化 CH 胺化在温和条件下(5 mol% 催化剂,60 °C)发生,并以适中的产率(29%-87%)提供吡咯烷产物,ee 适中(高达 73%)。在 4-芳基位置上具有 3,5-二烷基取代的底物使观察到的对映选择性最大化。使用 1H 和 19F NMR 光谱进行动力学研究以探测反应机理。一个小的分子间动力学同位素效应 (1.35 ± 0.03) 表明 H 原子提取步骤具有不对称过渡态,而反应速率测量为催化剂中的一级和底物浓度的零级。对映特异性氘标记研究表明,由于自由基旋转和 CN 键形成之间的可比速率,对映选择性由 H 原子提取和自由基重组步骤决定。此外,两步反应的竞争元素,其中从 pro-R 构型中去除 H 是优选的,而碳自由基的 Si 面发生优先自由基捕获可能导致观察到的 ee 减少,如理论计算所证实的那样。基于这些对映确定步骤,2,5-二叔吡咯烷的催化对映选择性合成被证明具有良好的收率(50-78%)和中等的ee(高达79%)。
更新日期:2021-01-04
中文翻译:

阴离子双恶唑啉 (BOX) 配体负载的镍亚胺基配合物催化的对映选择性 C-H 胺化
将三苯甲基取代的双恶唑啉 (TrHBOX) 制备为先前报道的能够进行闭环胺化催化的联吡啶镍系统的手性类似物。用二价 NiI2(py)4 进行配体金属化,然后还原钾石墨,得到一价 (TrHBOX)Ni(py) (4)。将 1.4 equiv 的 1-金刚基叠氮化物的苯溶液缓慢添加到 4 中生成四叠氮基 (TrHBOX)Ni(κ2-N4Ad2) (5) 和末端亚胺基加合物 (TrHBOX)Ni(NAd) (6)。通过单晶 X 射线晶体学、NMR 和 EPR 光谱学和计算对 6 进行的研究揭示了一种 Ni(II)-亚胺基自由基配方,类似于其双吡喃酮同系物。配合物 4 表现出对映选择性分子内 CH 键胺化,可从 4-aryl-2-methyl-2-azidopentanes 得到 N-杂环产物。催化 CH 胺化在温和条件下(5 mol% 催化剂,60 °C)发生,并以适中的产率(29%-87%)提供吡咯烷产物,ee 适中(高达 73%)。在 4-芳基位置上具有 3,5-二烷基取代的底物使观察到的对映选择性最大化。使用 1H 和 19F NMR 光谱进行动力学研究以探测反应机理。一个小的分子间动力学同位素效应 (1.35 ± 0.03) 表明 H 原子提取步骤具有不对称过渡态,而反应速率测量为催化剂中的一级和底物浓度的零级。对映特异性氘标记研究表明,由于自由基旋转和 CN 键形成之间的可比速率,对映选择性由 H 原子提取和自由基重组步骤决定。此外,两步反应的竞争元素,其中从 pro-R 构型中去除 H 是优选的,而碳自由基的 Si 面发生优先自由基捕获可能导致观察到的 ee 减少,如理论计算所证实的那样。基于这些对映确定步骤,2,5-二叔吡咯烷的催化对映选择性合成被证明具有良好的收率(50-78%)和中等的ee(高达79%)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号