Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-01-04 , DOI: 10.1016/j.tetlet.2020.152761 Yuxing Zhang , Xian-Rong Song , Fengyan Jin , Tao Yang , Ruchun Yang , Qiang Xiao
|
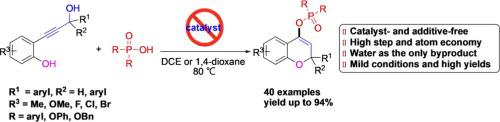
A novel, catalyst- and additive-free cascade phosphorylation/cyclization of readily available 2-propynolphenols with R2P(O)OH (R = aryl, OPh, OBn) was developed for the construction of 2H-chromen-4-yl phosphinates/phosphonates and water as the only byproduct under mild conditions. The reaction was achieved by using R2P(O)OH as acid-promoter, but also as the phosphinate source with the formation of phosphorylation allene intermediate, and followed by 6-endo-trig pathway. Additionally, this approach avoided the use of hazardous, air sensitive or activation reagents, and performed well with good functional group tolerance and satisfied yields.
中文翻译:

炔丙醇和R 2 P(O)OH的无催化剂和无添加剂级联磷酸化/环化
开发了一种新颖的,无催化剂和无添加剂的,易于获得的2-丙炔酚与R 2 P(O)OH(R =芳基,OPh,OBn)的级联磷酸化/环化反应,用于构建2 H -chromen-4-yl次膦酸酯/膦酸酯和水是在温和条件下唯一的副产物。通过使用R 2 P(O)OH作为酸促进剂,但也作为次膦酸酯源并形成磷酸化丙二烯中间体,并随后进行6-内-trig途径来实现反应。另外,该方法避免了使用危险的,对空气敏感的或活化的试剂,并且以良好的官能团耐受性和令人满意的产率表现良好。































 京公网安备 11010802027423号
京公网安备 11010802027423号