Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2021-01-04 , DOI: 10.1016/j.jclepro.2021.125807 Yanping Cui , Zhengwei Zhou , Ya Gao , Lidan Lei , Jungang Cao , Ruojie Wu , Lili Liang , Zhaoqiang Huang
|
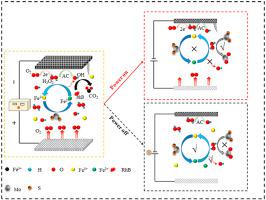
Without external O2 supply and solution pH adjusting, commercial molybdenum sulfide (MoS2) was firstly applied as co-catalyst in intermittent electro-Fenton process for Rhodamine B (RhB) degradation. In this energy saving system, Fe2+/Fe3+ conversion was facilitated and H2O2 activating was accelerated by MoS2. Granular activated carbon wrapped with stainless steel mesh (GACSS) was used as cathode, from which, H2O2 and iron sources were in-situ generated. With 0.1 g/L MoS2, and 50 mA applied current with 10 min on-10 min off power frequency, RhB degradation rate constant in the MoS2 intermittent electro-Fenton was approximately 2.4 times as that without MoS2 system. Quenching experiments, electron spin resonance (ESR) characterization and free radical fluorescence tests demonstrated hydroxyl radicals (·OH) was the dominant reactive species, and the superiority of intermittent electrolysis mode for RhB degradation. During power on, H2O2 was constantly generated on cathode. In electricity interval, Fe2+/Fe3+ conversion was accelerated on the active sites (Mo4+) to activate residual H2O2 and more ·OH was generated to RhB degradation. Additionally, the characterization analysis (XPS, SEM and FT-IR) demonstrated MoS2 and GACSS cathode exhibited excellent catalytic and chemical stability in recyclability. Low-cost multifunctional cathode with a high efficiency co-catalyst was combined in this energy saving electro-Fenton operating system for RhB degradation.
中文翻译:

节能的间歇式电子芬顿系统与商业化的MoS 2结合,可有效降解若丹明B
在无需外部O 2供给和溶液pH值调节的情况下,首先将商业化的硫化钼(MoS 2)用作间歇电芬顿工艺中的助催化剂,用于罗丹明B(RhB)的降解。在该节能系统中,促进了Fe 2+ / Fe 3+的转化,并且通过MoS 2促进了H 2 O 2的活化。用包裹有不锈钢网(GACSS)的颗粒状活性炭作为阴极,从中原位生成H 2 O 2和铁源。在0.1 g / L MoS 2和50 mA施加电流,10分钟通断频率和10分钟关断电源频率的情况下,MoS中RhB降解速率恒定2间歇式电子芬顿约为无MoS 2系统的2.4倍。淬火实验,电子自旋共振(ESR)表征和自由基荧光测试表明,羟基自由基(·OH)是主要的反应物种,并且间歇电解模式对RhB降解具有优越性。通电期间,在阴极上不断生成H 2 O 2。在电间隔中,Fe 2+ / Fe 3+在活性位点(Mo 4+)上加速转化,以活化残留的H 2 O 2。生成更多的·OH导致RhB降解。此外,表征分析(XPS,SEM和FT-IR)表明MoS 2和GACSS阴极在可回收性方面显示出出色的催化和化学稳定性。在这种节能的电子芬顿操作系统中结合了具有高效助催化剂的低成本多功能阴极,以降解RhB。

































 京公网安备 11010802027423号
京公网安备 11010802027423号