Electrochimica Acta ( IF 5.5 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.electacta.2020.137701 N Sethulakshmi , Subramanian Nellaiappan , Phanikumar Pentyala , Manu Sharma , Silvia Irusta , Parag A. Deshpande , Sudhanshu Sharma
|
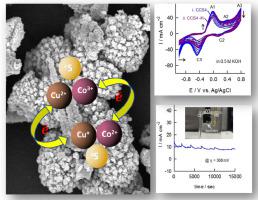
Understanding the electrocatalytic activity and redox mechanism in mixed metal sulfides has steered the present work. Mixed metal sulfide, CuCo2S4, synthesised by hydrothermal method, reveals the presence of highly crystalline nanocoral sheets of CuCo2S4 with mixed oxidation states of Co and Cu ions. Electrochemical measurements show superior oxygen evolution reaction (OER) activity and high exchange current density over CuCo2S4 in comparison with pristine (Co3S4 and Cu2S) and sintered CuCo2S4. In 0.5 M KOH, the nanocoral CuCo2S4 catalyst and its sintered form show a current density of 10 mA cm−2 at overpotential of 368 mV and 511 mV, respectively. A close inspection of the electrocatalytic performance in correlation with XPS spectra reveals the synergistic effect of Co and Cu ions through a redox interaction. Density Functional Theory calculations also indicate strong dependence of redox activity on redox couples as accompanied with changes in the partial charges of different constituent ions in the catalyst upon adsorption of hydroxyl ions which is a key step in OER. The study not only details sulfide-based catalytic compound for OER but also provides conclusive insights into the surface redox processes thereby providing an alternative direction to the search on novel OER catalysts.
中文翻译:

纳米珊瑚CuCo 2 S 4硫代松油:通过金属离子的氧化还原相互作用产生氧的反应
了解混合金属硫化物中的电催化活性和氧化还原机理已经指导了当前的工作。通过水热法合成的混合金属硫化物CuCo 2 S 4揭示了存在高结晶度的CoCo和Cu离子混合氧化态的CuCo 2 S 4纳米珊瑚薄片。电化学测量显示出优异的析氧反应(OER)的活性和高的交换电流密度超过CUCO 2小号4与原始(联合比较3小号4和Cu 2 S)和烧结CUCO 2小号4。在0.5 M KOH中,纳米珊瑚CuCo 2 S图4的催化剂及其烧结形式分别在368mV和511mV的超电势下显示出10mA cm -2的电流密度。仔细检查与XPS光谱相关的电催化性能,发现通过氧化还原相互作用,Co和Cu离子具有协同作用。密度泛函理论计算还表明,氧化还原活性对氧化还原对的强烈依赖性,伴随着吸附氢氧根离子后催化剂中不同组成离子的部分电荷的变化,这是OER的关键步骤。该研究不仅详细介绍了用于OER的基于硫化物的催化化合物,而且还提供了有关表面氧化还原过程的结论性见解,从而为新型OER催化剂的研究提供了替代方向。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号