当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyridine-2,6-dicarboxaldehyde-Enabled N-Terminal In Situ Growth of Polymer–Interferon α Conjugates with Significantly Improved Pharmacokinetics and In Vivo Bioactivity
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-12-31 , DOI: 10.1021/acsami.0c15786 Jiawei Sun 1 , Xinyu Liu 2, 3 , Jianwen Guo 1 , Wenguo Zhao 1 , Weiping Gao 2, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-12-31 , DOI: 10.1021/acsami.0c15786 Jiawei Sun 1 , Xinyu Liu 2, 3 , Jianwen Guo 1 , Wenguo Zhao 1 , Weiping Gao 2, 3
Affiliation
|
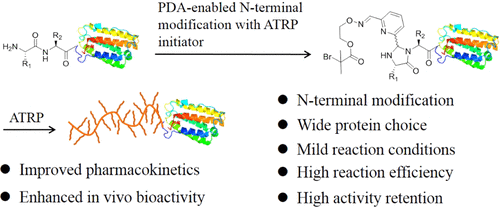
Polymer–protein conjugates are a class of biohybrids with unique properties that are highly useful in biomedicine ranging from protein therapeutics to biomedical imaging; however, it remains a considerable challenge to conjugate polymers to proteins in a site-specific, mild, and efficient way to form polymer–protein conjugates with uniform structures and properties and optimal functions. Herein we report pyridine-2,6-dicarboxaldehyde (PDA)-enabled N-terminal modification of proteins with polymerization initiators for in situ growth of poly(oligo(ethylene glycol)methyl ether methacrylate) (POEGMA) conjugates uniquely at the N-termini of a range of natural and recombinant proteins in a mild and efficient fashion. The formed POEGMA–protein conjugates showed highly retained in vitro bioactivity as compared with free proteins. Notably, the in vitro bioactivity of a POEGMA–interferon α (IFN) conjugate synthesized by this new chemistry is 8.1-fold higher than that of PEGASYS that is a commercially available and Food and Drug Administration (FDA) approved PEGylated IFN. The circulation half-life of the conjugate is similar to that of PEGASYS but is 46.2 times longer than that of free IFN. Consequently, the conjugate exhibits considerably improved antiviral bioactivity over free IFN and even PEGASYS in a mouse model. These results indicate that the PDA-enabled N-terminal grafting-from method is applicable to a number of proteins whose active sites are far away from the N-terminus for the synthesis of N-terminal polymer–protein conjugates with high yield, well-retained activity, and considerably improved pharmacology for biomedical applications.
中文翻译:

吡啶-2,6-二甲苯甲醛使N-端原位生长的聚合物-干扰素α具有显着改善的药代动力学和体内生物活性。
聚合物-蛋白质结合物是一类具有独特特性的生物杂交物,在蛋白质治疗到生物医学成像等生物医学中非常有用。然而,以位点特异性,温和有效的方式将聚合物与蛋白质偶联以形成具有均匀结构,特性和最佳功能的聚合物-蛋白质偶联物仍然是一个巨大的挑战。在这里,我们报告吡啶-2,6-二甲醛(PDA)使能N末端修饰的蛋白质与聚合引发剂在N-末端独特地原位生长聚(乙二醇(乙二醇)甲基醚甲基丙烯酸酯)(POEGMA)共轭物。以温和有效的方式制备一系列天然和重组蛋白。与游离蛋白相比,形成的POEGMA-蛋白结合物具有高度保留的体外生物活性。值得注意的是 通过这种新化学方法合成的POEGMA-干扰素α(IFN)偶联物的体外生物活性比市售的PEGASYS和食品和药物管理局(FDA)批准的PEG化IFN的生物活性高8.1倍。缀合物的循环半衰期与PEGASYS相似,但比游离IFN长46.2倍。因此,该缀合物在小鼠模型中比游离IFN甚至PEGASYS表现出显着改善的抗病毒生物活性。这些结果表明,启用PDA的N末端嫁接方法适用于许多蛋白质的活性位点远离N末端,从而可以高产率,高收率地合成N末端聚合物-蛋白质共轭物。保留活性,并大大改善了生物医学应用的药理学。
更新日期:2021-01-13
中文翻译:

吡啶-2,6-二甲苯甲醛使N-端原位生长的聚合物-干扰素α具有显着改善的药代动力学和体内生物活性。
聚合物-蛋白质结合物是一类具有独特特性的生物杂交物,在蛋白质治疗到生物医学成像等生物医学中非常有用。然而,以位点特异性,温和有效的方式将聚合物与蛋白质偶联以形成具有均匀结构,特性和最佳功能的聚合物-蛋白质偶联物仍然是一个巨大的挑战。在这里,我们报告吡啶-2,6-二甲醛(PDA)使能N末端修饰的蛋白质与聚合引发剂在N-末端独特地原位生长聚(乙二醇(乙二醇)甲基醚甲基丙烯酸酯)(POEGMA)共轭物。以温和有效的方式制备一系列天然和重组蛋白。与游离蛋白相比,形成的POEGMA-蛋白结合物具有高度保留的体外生物活性。值得注意的是 通过这种新化学方法合成的POEGMA-干扰素α(IFN)偶联物的体外生物活性比市售的PEGASYS和食品和药物管理局(FDA)批准的PEG化IFN的生物活性高8.1倍。缀合物的循环半衰期与PEGASYS相似,但比游离IFN长46.2倍。因此,该缀合物在小鼠模型中比游离IFN甚至PEGASYS表现出显着改善的抗病毒生物活性。这些结果表明,启用PDA的N末端嫁接方法适用于许多蛋白质的活性位点远离N末端,从而可以高产率,高收率地合成N末端聚合物-蛋白质共轭物。保留活性,并大大改善了生物医学应用的药理学。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号