Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-12-31 , DOI: 10.1016/j.jechem.2020.12.009 Mingxue Tang , Nhat N. Bui , Jin Zheng , Likai Song , Yan-Yan Hu
|
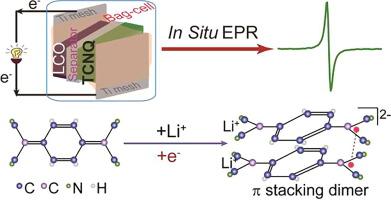
Organic electrodes are advantageous for rechargeable lithium-ion batteries owing to their high theoretical capacities, diverse functionalities, and environmental compatibility. Understanding the working mechanism of organic electrodes is vital to strategic materials design. However, due to lack of suitable characterization tools, it has been challenging to probe the reaction processes of organic electrodes in real-time. Here, non-destructive in situ electron paramagnetic resonance (EPR) was performed on a model organic electrode, 7,7,8,8-tetracyanoquinodimethane (TCNQ) used in rechargeable lithium-ion batteries, to directly follow the redox reactions in real-time. In order to minimize interfering signals from other parts of the batteries than the TCNQ electrode of interest, two sets of batteries are fabricated and studied with in situ EPR: (1) a LiCoO2//Li4Ti5O12 full-cell battery to determine the EPR signal evolution of additives and electrolytes; (2) a LiCoO2//TCNQ battery, and the difference in the observed EPR signals reflects purely the redox reactions of TCNQ upon lithiation and delithiation. A two-electron reversible redox reaction is delineated for TCNQ. TCNQ dimers form during the first electron injection upon lithiation and followed by the break-down of the dimers and associated electron coupling to produce massive delocalized electrons, resulting in increased EPR signal during the 2nd electron injection. Reversible trends are observed during electron ejection upon delithiation. In situ EPR is very sensitive to electron activities, thus is a powerful tool to follow redox reactions of organic electrodes, allowing for improved fundamental understanding of how organic electrodes work and for informed design of high-performance organic materials for energy storage.
中文翻译:

通过原位EPR实时监测有机电极7,7,8,8-四氰基喹二甲烷中的锂化过程
有机电极由于其高理论容量,多种功能和环境相容性,因此对于可再充电锂离子电池是有利的。了解有机电极的工作机制对于战略材料设计至关重要。然而,由于缺乏合适的表征工具,实时探测有机电极的反应过程一直是一项挑战。在这里,非破坏性的原位对可充电锂离子电池中使用的模型有机电极7,7,8,8-四氰基喹二甲烷(TCNQ)进行电子顺磁共振(EPR),以直接实时实时跟踪氧化还原反应。为了最大程度地减少除目标TCNQ电极以外的电池其他部分产生的干扰信号,使用原位EPR制作和研究了两组电池:(1)LiCoO 2 // Li 4 Ti 5 O 12全电池确定添加剂和电解质的EPR信号演变;(2)LiCoO 2// TCNQ电池,观察到的EPR信号的差异完全反映了TCNQ在锂化和脱锂时的氧化还原反应。描绘了TCNQ的两电子可逆氧化还原反应。TCNQ二聚体在锂化后的第一次电子注入过程中形成,随后二聚体的分解和相关的电子耦合产生大量的离域电子,从而导致第二次电子注入过程中EPR信号增加。在脱锂过程中的电子喷射过程中观察到可逆趋势。原位 EPR对电子活动非常敏感,因此是跟踪有机电极的氧化还原反应的有力工具,可以使人们更好地了解有机电极的工作原理,并明智地设计用于储能的高性能有机材料。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号