Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-12-31 , DOI: 10.1016/j.bmc.2020.115985 Shu-Yi Hao 1 , Zhi-Yuan Qi 1 , Shuai Wang 1 , Xing-Rong Wang 1 , Shi-Wu Chen 1
|
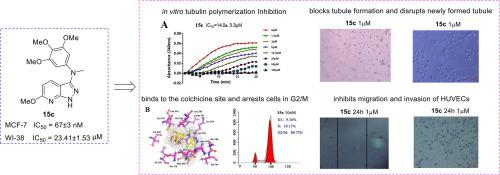
A new series of N-(3,4,5-trimethoxyphenyl)-1H-pyrazolo[3,4-b]pyridin-3-amine derivatives as tubulin polymerization inhibitors were synthesized, and evaluated for the anti-proliferative activities. A structure–activity relationship study revealed that the free amino moiety of 1H-pyrazolo[3,4-b]pyridin-3-amine played an essential role in the anti-proliferative activities. Especially, compound 15c displayed the strongest anti-proliferation against MCF-7 cells with IC50 value of 0.067 ± 0.003 μM, and high selectivity over the normal human embryonic lung WI-38 cells with IC50 value of 23.41 ± 1.53 μM. Further mechanistic studies revealed that 15c showed strong anti-tubulin polymerization activity, changed the morphology of tubulin, and arrested the cell cycle at the G2/M transition in MCF-7 cells. Molecular docking analysis suggested that 15c well occupied the colchicine-binding pocket of tubulin. Additionally, 15c demonstrated anti-angiogenic activities with blocking the migration, invasion and tube formation, disrupting the newly formed tube, and regulating both MMP-9 and TIMP-1 in HUVEC cells. In summary, our results highlight that compound 15c is a potential antitumor compound that are worthy of further development.
中文翻译:

作为具有抗血管生成作用的微管蛋白聚合抑制剂的 N-(3,4,5-三甲氧基苯基)-1H-吡唑并[3,4-b]吡啶-3-胺的合成和生物评价
合成了一系列新的N- (3,4,5-三甲氧基苯基) -1H-吡唑并[3,4- b ]吡啶-3-胺衍生物作为微管蛋白聚合抑制剂,并评价了其抗增殖活性。构效关系研究表明,1 H- pyrazolo[3,4- b ]pyridin-3-amine的游离氨基部分在抗增殖活性中起重要作用。特别是,化合物15c对 MCF-7 细胞显示出最强的抗增殖作用,IC 50值为 0.067 ± 0.003 μM,并且对正常人胚胎肺 WI-38 细胞具有高选择性,IC 50值 23.41 ± 1.53 μM。进一步的机理研究表明,15c显示出很强的抗微管蛋白聚合活性,改变了微管蛋白的形态,并在 MCF-7 细胞的 G2/M 转换时阻止了细胞周期。分子对接分析表明15c很好地占据了微管蛋白的秋水仙碱结合口袋。此外,15c显示出抗血管生成活性,可阻断迁移、侵袭和管形成,破坏新形成的管,并调节 HUVEC 细胞中的 MMP-9 和 TIMP-1。总之,我们的结果强调化合物15c是一种潜在的抗肿瘤化合物,值得进一步开发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号