Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-12-30 , DOI: 10.1016/j.jhazmat.2020.124927 Pankaj Bhatt 1 , Tushar Joshi 2 , Kalpana Bhatt 3 , Wenping Zhang 1 , Yaohua Huang 1 , Shaohua Chen 1
|
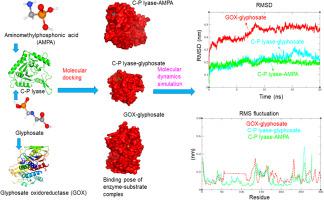
Widespread application of glyphosate poses a threat to living organisms. Microbial strains are able to degrade glyphosate via contrasting metabolic pathways with the help of enzymes. Glyphosate oxidoreductase (GOX) and C–P lyase are the key enzymes for the biodegradation of glyphosate and its intermediate metabolite aminomethylphosphonic acid (AMPA) in microbes. The microbial degradation of glyphosate has been reported, but the underlying molecular mechanism is still unclear. Therefore, in this study, the interaction mechanism of GOX and C–P lyase with glyphosate and AMPA were investigated by using molecular docking and molecular dynamics (MD) simulations. The results indicate that glyphosate contacts with the active site of GOX and C–P lyase by hydrogen bonds as well as hydrophobic and van der Waals interactions in aqueous solution to maintain its stability. The presence of glyphosate and AMPA in the active site significantly changes the conformation of GOX and C–P lyase. The results of the MD simulations confirm that GOX and C–P lyase complexes are stable during the catalytic reaction. This study offers a molecular level of understanding of the expression and function of GOX and C–P lyase for the bioremediation of glyphosate.
中文翻译:

草甘膦与草甘膦氧化还原酶和C-P裂解酶的结合相互作用:分子对接和分子动力学模拟研究
草甘膦的广泛应用对生物体构成威胁。微生物菌株能够在酶的帮助下通过对比代谢途径降解草甘膦。草甘膦氧化还原酶(GOX)和C-P裂解酶是微生物中草甘膦及其中间代谢产物氨甲基膦酸(AMPA)生物降解的关键酶。草甘膦的微生物降解已有报道,但潜在的分子机制仍不清楚。因此,本研究通过分子对接和分子动力学(MD)模拟研究了GOX和C-P裂解酶与草甘膦和AMPA的相互作用机制。结果表明,草甘膦在水溶液中通过氢键以及疏水性和范德华相互作用与GOX和C-P裂解酶的活性位点接触,以保持其稳定性。活性位点中草甘膦和 AMPA 的存在显着改变了 GOX 和 C-P 裂合酶的构象。 MD模拟结果证实GOX和C-P裂解酶复合物在催化反应过程中是稳定的。这项研究为草甘膦生物修复中 GOX 和 C-P 裂解酶的表达和功能提供了分子水平的理解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号