当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.oprd.0c00398 Jaka Orehek 1, 2 , Dušan Teslić 2 , Blaž Likozar 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.oprd.0c00398 Jaka Orehek 1, 2 , Dušan Teslić 2 , Blaž Likozar 1
Affiliation
|
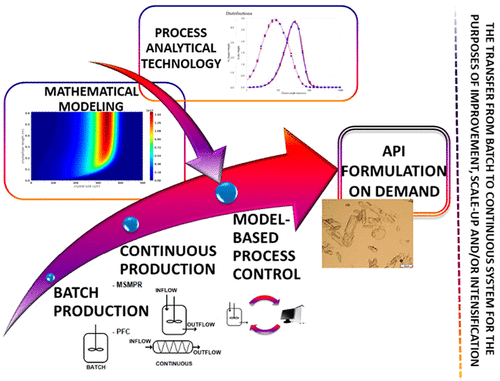
This scientific paper presents an overview of continuous solution crystallization in the pharmaceutical industry. Since the specific knowledge spectrum of precipitation is very broad, topics covering the following were analyzed and assessed in detail: a comparison between batch and continuous crystallizers, their engineering advantages/disadvantages, the introduction of the solid form structuring to continuously oriented chemical production, and the usage of the online process analytical technology (PAT) methodologies in interacting functional systems. Furthermore, mechanistic multiscale modeling, whose understanding is of decisive importance for further control application development, was thoroughly discussed as well. The multiscale modeling section also includes the simulation of processes and the optimization of crystallizers. In the last section, model-based predictive control (MPC) is considered, which also covers the intensification approach methods in the field of crystal formation and the introduction of new, highly potential continuous systems, e.g. the coiled flow inverter (CFI) crystallizer, in industry and academia. In the mentioned main sections, individual theme-related articles are collected, discussed, and compared; therefore, a reader can get acquainted with pieces of research, their found results, which have been developed until now, and various emerging perspectives. With regard to the importance of crystallization in the pharmaceutical industry, a reader can already find literature on continuous crystallization. However, due to the growing tendency toward the synthesis of products with specific properties which cannot be achieved with conventional approaches, more attention has been paid in the present work to the establishment of continuous systems for the production of specific fine chemicals. In addition, continuous crystallization could also enable more efficient and “on-demand” production, modeling for optimization, MPC, and intensification approaches. A review of the literature classifying studies according to the choice of a continuous system and their description was omitted in this study, as such reviews are already available in the literature.
中文翻译:

制药生产中的连续结晶过程:综述
该科学论文概述了制药行业中的连续溶液结晶。由于沉淀的具体知识范围非常广泛,因此对以下主题进行了详细分析和评估:分批结晶器和连续结晶器之间的比较,它们在工程上的优点/缺点,将固体形式的结构引入连续取向的化学生产中以及在线过程分析技术(PAT)方法在交互功能系统中的使用。此外,对机械多尺度建模的理解对于进一步控制应用程序的开发具有决定性的意义,也进行了全面讨论。多尺度建模部分还包括过程仿真和结晶器优化。在最后一部分中,考虑了基于模型的预测控制(MPC),它还涵盖了晶体形成领域的强化方法以及新的,潜力巨大的连续系统的引入,例如盘流逆变器(CFI)结晶器,在工业和学术界。在提到的主要部分中,收集,讨论和比较了与主题相关的各个文章;因此,读者可以熟悉研究,发现的结果(迄今为止已经开发)以及各种新兴的观点。关于结晶在制药工业中的重要性,读者已经可以找到有关连续结晶的文献。然而,由于合成具有日益增长的具有常规方法无法实现的具有特定性能的产物的趋势,因此在本工作中已经更加关注建立用于生产特定精细化学品的连续系统。此外,连续结晶还可以提高效率和按需生产,优化建模,MPC和强化方法。根据连续系统的选择,对文献分类研究的评论及其描述在本研究中被省略,因为此类评论已在文献中获得。
更新日期:2021-01-16
中文翻译:

制药生产中的连续结晶过程:综述
该科学论文概述了制药行业中的连续溶液结晶。由于沉淀的具体知识范围非常广泛,因此对以下主题进行了详细分析和评估:分批结晶器和连续结晶器之间的比较,它们在工程上的优点/缺点,将固体形式的结构引入连续取向的化学生产中以及在线过程分析技术(PAT)方法在交互功能系统中的使用。此外,对机械多尺度建模的理解对于进一步控制应用程序的开发具有决定性的意义,也进行了全面讨论。多尺度建模部分还包括过程仿真和结晶器优化。在最后一部分中,考虑了基于模型的预测控制(MPC),它还涵盖了晶体形成领域的强化方法以及新的,潜力巨大的连续系统的引入,例如盘流逆变器(CFI)结晶器,在工业和学术界。在提到的主要部分中,收集,讨论和比较了与主题相关的各个文章;因此,读者可以熟悉研究,发现的结果(迄今为止已经开发)以及各种新兴的观点。关于结晶在制药工业中的重要性,读者已经可以找到有关连续结晶的文献。然而,由于合成具有日益增长的具有常规方法无法实现的具有特定性能的产物的趋势,因此在本工作中已经更加关注建立用于生产特定精细化学品的连续系统。此外,连续结晶还可以提高效率和按需生产,优化建模,MPC和强化方法。根据连续系统的选择,对文献分类研究的评论及其描述在本研究中被省略,因为此类评论已在文献中获得。











































 京公网安备 11010802027423号
京公网安备 11010802027423号