当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De Novo Design, Solution Characterization, and Crystallographic Structure of an Abiological Mn–Porphyrin-Binding Protein Capable of Stabilizing a Mn(V) Species
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-29 , DOI: 10.1021/jacs.0c10136
Samuel I Mann 1 , Animesh Nayak 2 , George T Gassner 3 , Michael J Therien 2 , William F DeGrado 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-29 , DOI: 10.1021/jacs.0c10136
Samuel I Mann 1 , Animesh Nayak 2 , George T Gassner 3 , Michael J Therien 2 , William F DeGrado 1
Affiliation
|
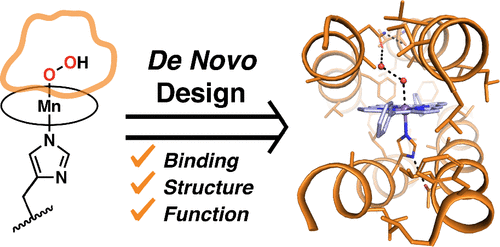
De novo protein design offers the opportunity to test our understanding of how metalloproteins perform difficult transformations. Attaining high-resolution structural information is critical to understanding how such designs function. There have been many successes in the design of porphyrin-binding proteins; however, crystallographic characterization has been elusive, limiting what can be learned from such studies as well as the extension to new functions. Moreover, formation of highly oxidizing high-valent intermediates poses design challenges that have not been previously implemented: (1) purposeful design of substrate/oxidant access to the binding site and (2) limiting deleterious oxidation of the protein scaffold. Here we report the first crystallographically characterized porphyrin-binding protein that was programmed to not only bind a synthetic Mn-porphyrin but also maintain binding site access to form high-valent oxidation states. We explicitly designed a binding site with accessibility to dioxygen units in the open coordination site of the Mn center. In solution, the protein is capable of accessing a high-valent Mn(V)-oxo species which can transfer an O atom to a thioether substrate. The crystallographic structure is within 0.6 Å of the design and indeed contained an aquo ligand with a second water molecule stabilized by hydrogen bonding to a Gln side chain in the active site, offering a structural explanation for the observed reactivity.
中文翻译:

能够稳定 Mn(V) 物种的非生物 Mn-卟啉结合蛋白的从头设计、溶液表征和晶体结构
从头蛋白质设计提供了检验我们对金属蛋白质如何进行困难转化的理解的机会。获得高分辨率的结构信息对于理解此类设计的功能至关重要。卟啉结合蛋白的设计取得了许多成功;然而,晶体学表征一直难以捉摸,限制了从此类研究中可以学到的东西以及新功能的扩展。此外,高氧化性高价中间体的形成带来了以前没有实施过的设计挑战:(1) 有目的地设计底物/氧化剂进入结合位点和 (2) 限制蛋白质支架的有害氧化。在这里,我们报告了第一个晶体学特征的卟啉结合蛋白,该蛋白被编程为不仅可以结合合成的 Mn-卟啉,还可以保持结合位点的访问以形成高价氧化态。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。
更新日期:2020-12-29
中文翻译:

能够稳定 Mn(V) 物种的非生物 Mn-卟啉结合蛋白的从头设计、溶液表征和晶体结构
从头蛋白质设计提供了检验我们对金属蛋白质如何进行困难转化的理解的机会。获得高分辨率的结构信息对于理解此类设计的功能至关重要。卟啉结合蛋白的设计取得了许多成功;然而,晶体学表征一直难以捉摸,限制了从此类研究中可以学到的东西以及新功能的扩展。此外,高氧化性高价中间体的形成带来了以前没有实施过的设计挑战:(1) 有目的地设计底物/氧化剂进入结合位点和 (2) 限制蛋白质支架的有害氧化。在这里,我们报告了第一个晶体学特征的卟啉结合蛋白,该蛋白被编程为不仅可以结合合成的 Mn-卟啉,还可以保持结合位点的访问以形成高价氧化态。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。我们在 Mn 中心的开放协调站点中明确设计了一个可访问双氧单元的绑定站点。在溶液中,该蛋白质能够获得高价 Mn(V)-oxo 物种,后者可以将 O 原子转移到硫醚底物上。晶体结构在设计的 0.6 Å 范围内,并且确实包含一个水配体和第二个水分子,通过氢键与活性位点的 Gln 侧链稳定,为观察到的反应性提供了结构解释。

































 京公网安备 11010802027423号
京公网安备 11010802027423号