当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assembly of α‐(Hetero)aryl Nitriles via Copper‐Catalyzed Coupling Reactions with (Hetero)aryl Chlorides and Bromides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-28 , DOI: 10.1002/anie.202014638 Dawei Ma 1 , Ying Chen 2 , Lanting Xu 3 , Yongwen Jiang 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-28 , DOI: 10.1002/anie.202014638 Dawei Ma 1 , Ying Chen 2 , Lanting Xu 3 , Yongwen Jiang 3
Affiliation
|
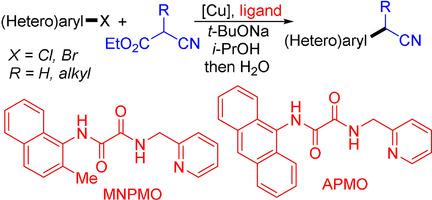
α‐(Hetero)aryl nitriles are important structural motifs for pharmaceutical design. The known methods for direct synthesis of these compounds via coupling with (hetero)aryl halides suffer from narrow reaction scope. Herein, we report that the combination of copper salts and oxalic diamides enables the coupling of a variety of (hetero)aryl halides (Cl, Br) and ethyl cyanoacetate under mild conditions, affording α‐(hetero)arylacetonitriles via one‐pot decarboxylation. Additionally, the CuBr/oxalic diamide catalyzed coupling of (hetero)aryl bromides with α‐alkyl‐substituted ethyl cyanoacetates proceeds smoothly at 60 °C, leading to the formation of α‐alkyl (hetero)arylacetonitriles after decarboxylation. The method features a general substrate scope and is compatible with various functionalities and heteroaryls.
中文翻译:

通过铜催化的(杂)芳基氯化物和溴化物的偶联反应组装α-(杂)芳基腈
α-(杂)芳基腈是药物设计的重要结构图案。通过与(杂)芳基卤化物偶合直接合成这些化合物的已知方法具有狭窄的反应范围。本文中,我们报道铜盐和草酸二酰胺的结合能够在温和的条件下偶联多种(杂)芳基卤化物(Cl,Br)和氰基乙酸乙酯,从而通过一锅脱羧作用提供α-(杂)芳基乙腈。此外,(杂)芳基溴化物与α-烷基取代的氰基乙酸乙酯的CuBr /草酸二酰胺催化偶合在60°C下平稳进行,导致脱羧后形成α-烷基(杂)芳基乙腈。该方法具有一般的底物范围,并且与各种功能和杂芳基相容。
更新日期:2020-12-28
中文翻译:

通过铜催化的(杂)芳基氯化物和溴化物的偶联反应组装α-(杂)芳基腈
α-(杂)芳基腈是药物设计的重要结构图案。通过与(杂)芳基卤化物偶合直接合成这些化合物的已知方法具有狭窄的反应范围。本文中,我们报道铜盐和草酸二酰胺的结合能够在温和的条件下偶联多种(杂)芳基卤化物(Cl,Br)和氰基乙酸乙酯,从而通过一锅脱羧作用提供α-(杂)芳基乙腈。此外,(杂)芳基溴化物与α-烷基取代的氰基乙酸乙酯的CuBr /草酸二酰胺催化偶合在60°C下平稳进行,导致脱羧后形成α-烷基(杂)芳基乙腈。该方法具有一般的底物范围,并且与各种功能和杂芳基相容。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号