Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-12-29 , DOI: 10.1016/j.jcat.2020.12.012 Aiping Jia , Yunshang Zhang , Tongyang Song , Zhenhua Zhang , Cen Tang , Yiming Hu , Wanbin Zheng , Mengfei Luo , Jiqing Lu , Weixin Huang
|
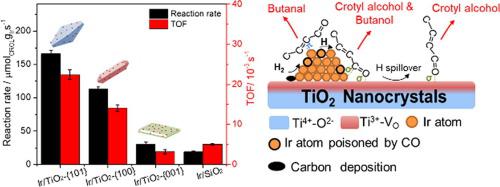
Supported Ir/TiO2 catalysts with the anatase TiO2 nanocrystals exposing {1 0 1}, {1 0 0} and {0 0 1} planes were tested for selective hydrogenation of crotonaldehyde. The Ir/TiO2-{1 0 1} catalyst dominantly exposing {1 0 1} plane gave a initial reaction rate of 166.1 μmol gIr-1 s−1 at 80 °C and a turnover frequency of crotyl alcohol formation of 0.022 s−1, which is 5 and 7 times respectively higher than those obtained on the Ir/TiO2-{0 0 1} catalyst dominantly exposing {0 0 1} plane even though they have similar Ir particle sizes (ca. 1.3 nm). The reaction behaviors were related to the surface oxygen vacancy, which served as surface acid sites and provide adsorption sites for C O bond in the CRAL molecule. Therefore, the higher concentration of oxygen vacancy in the Ir/TiO2-{1 0 1} accounted for the improved performance compared to the Ir/TiO2-{0 0 1}. However, strong adsorption of crotonaldehyde and/or products on the catalyst surface, as well as the CO poisoning via decarbonylation of crotonaldehyde, were the main reasons for the catalyst deactivation.
O bond in the CRAL molecule. Therefore, the higher concentration of oxygen vacancy in the Ir/TiO2-{1 0 1} accounted for the improved performance compared to the Ir/TiO2-{0 0 1}. However, strong adsorption of crotonaldehyde and/or products on the catalyst surface, as well as the CO poisoning via decarbonylation of crotonaldehyde, were the main reasons for the catalyst deactivation.
中文翻译:

锐钛矿型TiO 2在Ir / TiO 2催化剂上对巴豆醛选择性加氢的晶面效应
测试了具有暴露{1 0 1},{1 0 0}和{0 0 1}平面的锐钛矿型TiO 2纳米晶体的负载Ir / TiO 2催化剂对巴豆醛的选择性加氢作用。主要暴露于{1 0 1}平面的Ir / TiO 2- {1 0 1}催化剂在80°C下的初始反应速率为166.1μmolg Ir -1 s -1,巴豆醇形成的周转频率为0.022 s -1,分别比在Ir / TiO 2上获得的高-1和-1-{0 0 1}催化剂主要暴露{0 0 1}平面,即使它们具有相似的Ir粒径(约1.3 nm)也是如此。反应行为与表面氧空位有关,表面氧空位充当表面酸位点,并为 CRAL分子中的C O键提供吸附位点。因此,与Ir / TiO 2- {0 0 1}相比,Ir / TiO 2- {1 0 1}中较高的氧空位浓度导致性能提高。然而,巴豆醛和/或产物在催化剂表面上的强吸附以及巴豆醛脱羰引起的CO中毒是催化剂失活的主要原因。
CRAL分子中的C O键提供吸附位点。因此,与Ir / TiO 2- {0 0 1}相比,Ir / TiO 2- {1 0 1}中较高的氧空位浓度导致性能提高。然而,巴豆醛和/或产物在催化剂表面上的强吸附以及巴豆醛脱羰引起的CO中毒是催化剂失活的主要原因。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号