Chemosphere ( IF 8.1 ) Pub Date : 2020-12-27 , DOI: 10.1016/j.chemosphere.2020.129436 Meng Xiao , Yanfeng Qi , Qingmin Feng , Kun Li , Kaiqi Fan , Tingting Huang , Pei Qu , Hengjun Gai , Hongbing Song
|
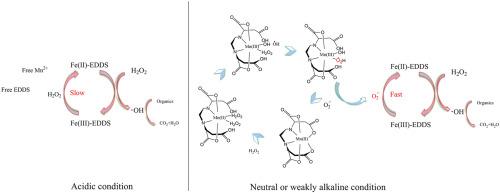
P-cresol is a highly toxic phenolic pollutant in coal chemical wastewater. The effective removal of p-cresol is of great significance to the ecological environment. In this study, the degradation of p-cresol by the Fe(III)-EDDS/H2O2 Fenton-like reaction modified by Mn2+ was investigated. The results showed that the removal rate of p-cresol could be significantly increased by the addition of Mn2+ under neutral and weakly alkaline conditions (pH 6.5–8.5). Acidic conditions (pH 3.5) were not conducive to the Fenton-like reaction. This is because a neutral or weakly alkaline environment is conducive to Mn2+-EDDS complex formation, which can produce O2·- to accelerate the reduction of Fe(III), and the efficiency of p-cresol degradation through a Fenton-like reaction catalyzed by the Fe(III)-EDDS complex is significantly improved. In addition, the degradation of EDDS through ·OH was reduced by O2·-, which maintained and stabilized the Mn2+-EDDS complex and Fe(III)-EDDS complex. Under neutral conditions, the optimal dosage of Fe(III) is 0.7 mM, and the optimal molar ratios are EDDS/Fe(III) = 1: 1, Mn2+/Fe(III) = 1: 1, and H2O2/Fe(III) = 15: 1. The addition of free radical clearance isopropanol and CHCl3 proved that ·OH was the main active substance in the p-cresol degradation process.
中文翻译:

锰离子促进Fe(III)-EDDS / H 2 O 2芬顿样反应降解对甲酚:pH值和反应机理的影响
对甲酚是煤化工废水中的一种剧毒的酚类污染物。有效去除对甲酚对生态环境具有重要意义。在这项研究中,研究了由Mn 2+修饰的Fe(III)-EDDS / H 2 O 2 Fenton样反应对对甲酚的降解。结果表明,在中性和弱碱性条件下(pH 6.5-8.5),通过添加Mn 2+可以显着提高对甲酚的去除率。酸性条件(pH 3.5)不利于类芬顿反应。这是因为中性或弱碱性环境有利于Mn 2+ -EDDS络合物的形成,从而生成O 2 ·-加速Fe(III)的还原,并显着提高了通过Fe(III)-EDDS络合物催化的Fenton样反应对对甲酚的降解效率。此外,O 2 ·-减少了EDDS通过·OH的降解,从而维持并稳定了Mn 2+ -EDDS络合物和Fe(III)-EDDS络合物。在中性条件下,Fe(III)的最佳添加量为0.7 mM,最佳摩尔比为EDDS / Fe(III)= 1、1,Mn 2+ / Fe(III)= 1:1和H 2 O 2 / Fe(III)= 15:1.自由基清除率的异丙醇和CHCl 3的添加证明·OH是对甲酚降解过程中的主要活性物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号