当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficiency of Enzymatic O2 Reduction by Myrothecium verrucaria Bilirubin Oxidase Probed by Surface Plasmon Resonance, PMIRRAS, and Electrochemistry
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-07-25 00:00:00 , DOI: 10.1021/acscatal.6b01423
Cristina Gutierrez-Sanchez 1 , Alexandre Ciaccafava 2 , Pierre Yves Blanchard 1 , Karen Monsalve 1 , Marie Thérèse Giudici-Orticoni 1 , Sophie Lecomte 3 , Elisabeth Lojou 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-07-25 00:00:00 , DOI: 10.1021/acscatal.6b01423
Cristina Gutierrez-Sanchez 1 , Alexandre Ciaccafava 2 , Pierre Yves Blanchard 1 , Karen Monsalve 1 , Marie Thérèse Giudici-Orticoni 1 , Sophie Lecomte 3 , Elisabeth Lojou 1
Affiliation
|
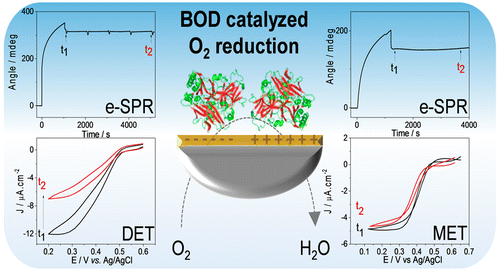
Deciphering which parameters control the immobilization of enzymes on solid supports is essential for the development of biotechnological devices such as biosensors, bioreactors, and enzymatic fuel cells. In this work, we used surface plasmon resonance (SPR) coupled with electrochemistry and polarization modulated infrared reflection absorption spectroscopy (PMIRRAS) to correlate the loading, the conformation, and the activity of Myrothecium verrucaria bilirubin oxidase (Mv BOD) enzymes immobilized on two oppositely charged self-assembled monolayers (SAMs) on gold electrodes. The SPR signal showed that an enzyme layer close to a monolayer was formed by spontaneous adsorption on both negatively and positively charged SAMs. A different catalytic process for O2 reduction was obtained, however, being a direct catalysis at negative interfaces and a mediated catalysis at positive interfaces, in relation to the charge of the amino acids surrounding the surface of the Cu T1 and the dipole moment direction of Mv BOD. The stability of the enzymatic current was dependent on the SAM type. On the positively charged SAM electrode, the mediated catalytic current was stable with time. On the negatively charged SAM electrode, the direct catalytic current decreased continuously with time, leading to a decrease in the TOF (turnover frequency) from 114 to 7 s–1, while the SPR signal remained stable, showing that the decrease in the catalytic current is not related to a desorption process. PMIRRAS studies suggested a conformational change in the tertiary structure as a result of strong electrostatic interactions between arginine residues close to the T1 Cu and the carboxylic functions on the SAM. Covalent binding, however, resulted in a great enhancement of the current stability, which can be explained by a rigidification of the enzyme layer.
中文翻译:

酶的O效率2还原漆斑疣胆红素氧化酶探索Sun Yatsen通过表面等离子体共振,PMIRRAS,和电化学
解释哪些参数控制酶在固体支持物上的固定化对于生物技术设备(例如生物传感器,生物反应器和酶促燃料电池)的开发至关重要。在这项工作中,我们使用了表面等离子体共振(SPR)结合电化学和偏振调制红外反射吸收光谱(PMIRRAS)来关联固定在两种相反方向上的疣粒疣绿胆红素氧化酶(Mv BOD)酶的负载,构象和活性。在金电极上带电的自组装单分子层(SAMs)。SPR信号表明,通过在负电荷和正电荷SAM上自发吸附形成了接近单层的酶层。O 2的不同催化过程然而,相对于Cu T1表面周围的氨基酸电荷和Mv BOD的偶极矩方向,获得的还原是在负界面处的直接催化和在正界面处的介导的催化。酶电流的稳定性取决于SAM类型。在带正电的SAM电极上,介导的催化电流随时间稳定。在带负电荷的SAM电极上,直接催化电流随时间连续下降,导致TOF(周转频率)从114降低到7 s –1,而SPR信号保持稳定,表明催化电流的减少与解吸过程无关。PMIRRAS研究表明,由于靠近T1 Cu的精氨酸残基与SAM上的羧基功能强大的静电相互作用,导致三级结构发生构象变化。然而,共价结合导致电流稳定性的极大提高,这可以通过酶层的硬化来解释。
更新日期:2016-07-25
中文翻译:

酶的O效率2还原漆斑疣胆红素氧化酶探索Sun Yatsen通过表面等离子体共振,PMIRRAS,和电化学
解释哪些参数控制酶在固体支持物上的固定化对于生物技术设备(例如生物传感器,生物反应器和酶促燃料电池)的开发至关重要。在这项工作中,我们使用了表面等离子体共振(SPR)结合电化学和偏振调制红外反射吸收光谱(PMIRRAS)来关联固定在两种相反方向上的疣粒疣绿胆红素氧化酶(Mv BOD)酶的负载,构象和活性。在金电极上带电的自组装单分子层(SAMs)。SPR信号表明,通过在负电荷和正电荷SAM上自发吸附形成了接近单层的酶层。O 2的不同催化过程然而,相对于Cu T1表面周围的氨基酸电荷和Mv BOD的偶极矩方向,获得的还原是在负界面处的直接催化和在正界面处的介导的催化。酶电流的稳定性取决于SAM类型。在带正电的SAM电极上,介导的催化电流随时间稳定。在带负电荷的SAM电极上,直接催化电流随时间连续下降,导致TOF(周转频率)从114降低到7 s –1,而SPR信号保持稳定,表明催化电流的减少与解吸过程无关。PMIRRAS研究表明,由于靠近T1 Cu的精氨酸残基与SAM上的羧基功能强大的静电相互作用,导致三级结构发生构象变化。然而,共价结合导致电流稳定性的极大提高,这可以通过酶层的硬化来解释。

































 京公网安备 11010802027423号
京公网安备 11010802027423号