Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-12-26 , DOI: 10.1016/j.cej.2020.128182 Wenze Guo , Zheng Zhang , Jasper Hacking , Hero Jan Heeres , Jun Yue
|
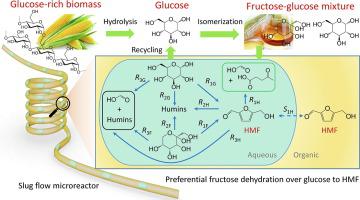
A two-step process combining the (equilibrium) glucose isomerization to fructose with selective dehydration of fructose in the obtained sugar mixture to 5-hydroxymethylfurfural (HMF), where glucose is largely unconverted and recycled, represents an attractive concept to increase the overall efficiency for HMF synthesis. This work presents experimental and modelling studies on the conversion of such fructose-glucose mixture to HMF using the sulfuric acid catalyst in a water-methyl isobutyl ketone biphasic system under a wide range of conditions (e.g., temperature, catalyst and sugar concentrations). Through detailed product analyses and ESI-MS spectroscopy, the excess formation of formic acid (together with humins) by the direct sugar/HMF degradation was confirmed and included in the reaction network (neglected in most literatures). The kinetic modelling based on batch experiments in monophasic water well describes the measurements thereof, whereas distinct deviations were found in the prediction of typical literature kinetic models. The incorporation of HMF equilibrium extraction into the developed kinetic model, with consideration of phase volume change as a function of temperature and partial phase miscibility, enables to predict reaction results in the biphasic system in batch. This kinetic model allows to optimize conditions for HMF synthesis that are favored in continuous reactors with minimized back mixing. Based on the model implications, the biphasic system was optimized with slug flow microreactors to better address process intensification and scale-up aspects. Using a simulated fructose-glucose mixture feedstock to represent commercially available high fructose corn syrups, a maximum HMF yield of 81% was obtained at 155 °C over 0.05 M H2SO4 at a residence time of 16 min in the microreactor, with 96% fructose conversion and over 95% of glucose remaining unconverted.
中文翻译:

双相体系中果糖-葡萄糖混合物在硫酸催化剂上由果糖-葡萄糖混合物选择性脱水成5-羟甲基糠醛的实验研究和动力学模型
分两步进行的过程(将葡萄糖的平衡异构化为果糖,然后将所获得的糖混合物中的果糖选择性脱水为5-羟甲基糠醛(HMF),其中葡萄糖大部分未转化并循环利用)代表了一个颇具吸引力的概念,可以提高糖的总效率HMF合成。这项工作提出了在水-甲基异丁基酮双相系统中,在广泛的条件(例如温度,催化剂和糖浓度)下使用硫酸催化剂将果糖-葡萄糖混合物转化为HMF的实验和模型研究。通过详细的产品分析和ESI-MS光谱分析,证实了糖/ HMF的直接降解会过量形成甲酸(与腐殖质一起),并将其包括在反应网络中(大多数文献中都忽略了)。在单相水井中基于批处理实验的动力学模型描述了其测量值,而在典型文献动力学模型的预测中发现了明显的偏差。将HMF平衡萃取结合到已开发的动力学模型中,并考虑相体积随温度和部分相混溶性的变化,从而可以批量预测两相系统中的反应结果。该动力学模型允许优化HMF合成的条件,这在连续反应器中有利于以最小的反混进行。基于模型的含义,双相系统使用团状流微反应器进行了优化,以更好地解决过程强度和规模扩大的问题。2 SO 4在微反应器中停留时间为16分钟,其中96%的果糖转化率和95%以上的葡萄糖未转化率。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号