当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introducing a Hydrogen‐Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexamethyldisilazides (MHMDS, M=Li, Na, K, Rb and Cs) with Ammonia
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-07-26 , DOI: 10.1002/chem.201600833
Roman Neufeld 1 , Reent Michel 1 , Regine Herbst-Irmer 1 , Ralf Schöne 1 , Dietmar Stalke 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-07-26 , DOI: 10.1002/chem.201600833
Roman Neufeld 1 , Reent Michel 1 , Regine Herbst-Irmer 1 , Ralf Schöne 1 , Dietmar Stalke 1
Affiliation
|
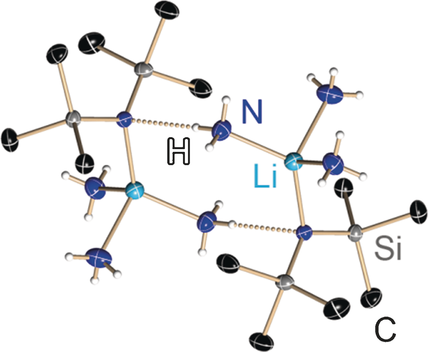
Alkali metal 1,1,1,3,3,3‐hexamethyldisilazide (MHMDSs) are one of the most utilised weakly nucleophilic Brønsted bases in synthetic chemistry and especially in natural product synthesis. Like lithium organics, they aggregate depending on the employed donor solvents. Thus, they show different reactivity and selectivity as a function of their aggregation and solvation state. To date, monomeric LiHMDS with monodentate donor bases was only characterised in solution. Since the first preparation of LiHMDS in 1959 by Wannagat and Niederprüm, all efforts to crystallise monomeric LiHMDS in the absence of chelating ligands failed. Herein, we present ammonia adducts of LiHMDS, NaHMDS, KHMDS, RbHMDS and CsHMDS with unprecedented aggregation motifs: 1) The hitherto missing monomeric key compound in the LiHMDS aggregation architectures. Monomeric crystal structures of trisolvated LiHMDS (1) and NaHMDS (2), showing unique intermolecular hydrogen bonds, 2) the unprecedented tetrasolvated KHMDS (3) and RbHMDS (4) dimers and 3) the disolvated CsHMDS (5) dimer with very close intermolecular Si−CH3⋅⋅⋅Cs s‐block “agostic” interactions have been prepared and characterised by single‐crystal X‐ray structure analysis.
中文翻译:

将氢键供体引入弱亲核的布朗斯台德碱:含氨的碱金属六甲基二硅叠氮化物(MHMDS,M = Li,Na,K,Rb和Cs)
碱金属1,1,1,3,3,3-六甲基二硅叠氮化物(MHMDSs)是合成化学,尤其是天然产物合成中最常用的弱亲核布朗斯台德碱之一。像锂有机物一样,它们会根据所使用的施主溶剂而聚集。因此,根据它们的聚集和溶剂化状态,它们显示出不同的反应性和选择性。迄今为止,仅在溶液中表征了具有单齿供体碱的单体LiHMDS。自1959年Wannagat和Niederprüm首次制备LiHMDS以来,在没有螯合配体的情况下使单体LiHMDS结晶的所有努力均告失败。在此,我们介绍具有前所未有的聚集基序的LiHMDS,NaHMDS,KHMDS,RbHMDS和CsHMDS的氨加合物:1)迄今为止在LiHMDS聚集体系中缺失的单体关键化合物。1)和加入NaHMDS(2),显示出独特的分子间氢键,2)前所未有tetrasolvated KHMDS(3)和RbHMDS(4)二聚体和3)disolvated CsHMDS(5)二聚体与非常接近的分子间的Si-CH 3 ⋅⋅⋅已经准备了Cs s嵌段的“声波”相互作用,并通过单晶X射线结构分析对其进行了表征。
更新日期:2016-07-26
中文翻译:

将氢键供体引入弱亲核的布朗斯台德碱:含氨的碱金属六甲基二硅叠氮化物(MHMDS,M = Li,Na,K,Rb和Cs)
碱金属1,1,1,3,3,3-六甲基二硅叠氮化物(MHMDSs)是合成化学,尤其是天然产物合成中最常用的弱亲核布朗斯台德碱之一。像锂有机物一样,它们会根据所使用的施主溶剂而聚集。因此,根据它们的聚集和溶剂化状态,它们显示出不同的反应性和选择性。迄今为止,仅在溶液中表征了具有单齿供体碱的单体LiHMDS。自1959年Wannagat和Niederprüm首次制备LiHMDS以来,在没有螯合配体的情况下使单体LiHMDS结晶的所有努力均告失败。在此,我们介绍具有前所未有的聚集基序的LiHMDS,NaHMDS,KHMDS,RbHMDS和CsHMDS的氨加合物:1)迄今为止在LiHMDS聚集体系中缺失的单体关键化合物。1)和加入NaHMDS(2),显示出独特的分子间氢键,2)前所未有tetrasolvated KHMDS(3)和RbHMDS(4)二聚体和3)disolvated CsHMDS(5)二聚体与非常接近的分子间的Si-CH 3 ⋅⋅⋅已经准备了Cs s嵌段的“声波”相互作用,并通过单晶X射线结构分析对其进行了表征。

































 京公网安备 11010802027423号
京公网安备 11010802027423号