当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversification of ortho‐Fused Cycloocta‐2,5‐dien‐1‐one Cores and Eight‐ to Six‐Ring Conversion by σ Bond C−C Cleavage
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-07-25 , DOI: 10.1002/chem.201601970 Lee Eccleshare 1 , Leticia Lozada-Rodríguez 1 , Phillippa Cooper 1 , Laurence Burroughs 1 , John Ritchie 1 , William Lewis 1 , Simon Woodward 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-07-25 , DOI: 10.1002/chem.201601970 Lee Eccleshare 1 , Leticia Lozada-Rodríguez 1 , Phillippa Cooper 1 , Laurence Burroughs 1 , John Ritchie 1 , William Lewis 1 , Simon Woodward 1
Affiliation
|
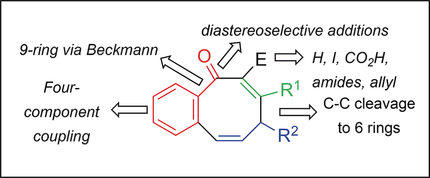
Sequential treatment of 2‐C6H4Br(CHO) with LiC≡CR1 (R1=SiMe3, tBu), nBuLi, CuBr⋅SMe2 and HC≡CCHClR2 [R2=Ph, 4‐CF3Ph, 3‐CNPh, 4‐(MeO2C)Ph] at −50 °C leads to formation of an intermediate carbanion (Z)‐1,2‐C6H4{CA(=O)C≡CBR1}{CH=CH(CH−)R2} (4). Low temperatures (−50 °C) favour attack at CB leading to kinetic formation of 6,8‐bicycles containing non‐classical C‐carbanion enolates (5). Higher temperatures (−10 °C to ambient) and electron‐deficient R2 favour retro σ‐bond C−C cleavage regenerating 4, which subsequently closes on CA providing 6,6‐bicyclic alkoxides (6). Computational modelling (CBS‐QB3) indicated that both pathways are viable and of similar energies. Reaction of 6 with H+ gave 1,2‐dihydronaphthalen‐1‐ols, or under dehydrating conditions, 2‐aryl‐1‐alkynylnaphthlenes. Enolates 5 react in situ with: H2O, D2O, I2, allylbromide, S2Me2, CO2 and lead to the expected C‐E derivatives (E=H, D, I, allyl, SMe, CO2H) in 49–64 % yield directly from intermediate 5. The parents (E=H; R1=SiMe3, tBu; R2=Ph) are versatile starting materials for NaBH4 and Grignard C=O additions, desilylation (when R1=SiMe) and oxime formation. The latter allows formation of 6,9‐bicyclics via Beckmann rearrangement. The 6,8‐ring iodides are suitable Suzuki precursors for Pd‐catalysed C−C coupling (81–87 %), whereas the carboxylic acids readily form amides under T3P® conditions (71–95 %).
中文翻译:

通过σ键C-C裂解使正交融合的Cycloocta-2,5-dien-1-one核和8到6环转换多样化
的2-C顺序处理6 ħ 4能Br(CHO)与LiC≡CR 1(R 1 =森达3,吨丁基),Ñ丁基锂,CuBr⋅SMe 2和HC≡CCHClR 2 [R 2 =苯基,4-CF 3 PH,3- CNPH,4-(MEO 2 C)PH]在-50℃下导致形成的中间碳负离子(的ž)-1,2--C 6 H ^ 4 {C阿(= O)C≡C乙- [R 1 } {CH = CH(CH -)R 2 }(4)。低温(−50°C)有利于C B的侵蚀导致6,8个自行车的动力学形成,其中包含非经典的C-碳双烯酸烯醇化物(5)。较高的温度(相对于环境温度为−10°C)和缺乏电子的R 2有利于逆向σ键C-C裂解再生4,其随后在C A上关闭,提供6,6-双环醇盐(6)。计算模型(CBS-QB3)表明这两种途径都是可行的,并且具有相似的能量。6与H +反应生成1,2-二氢萘-1-醇,或在脱水条件下生成2-芳基-1-炔基萘。烯醇盐5与以下物质原位反应:H 2 O,D 2 O,I 2,烯丙基溴,S 2 Me 2,CO 2并直接从中间体5产生49–64%的预期C- E衍生物(E = H,D,I,烯丙基,SMe,CO 2 H)。母体(E = H; R 1 = SiMe 3,t Bu; R 2 = Ph)是用于NaBH 4和格利雅德C = O的添加,脱甲硅烷基化的通用原料(当R 1时)= SiMe)和肟的形成。后者允许通过贝克曼重排形成6,9-双环。6,8环碘化物是适合Pd催化的C-C偶联的Suzuki前体(81-87%),而羧酸在T3P®条件下很容易形成酰胺(71-95%)。
更新日期:2016-07-25
中文翻译:

通过σ键C-C裂解使正交融合的Cycloocta-2,5-dien-1-one核和8到6环转换多样化
的2-C顺序处理6 ħ 4能Br(CHO)与LiC≡CR 1(R 1 =森达3,吨丁基),Ñ丁基锂,CuBr⋅SMe 2和HC≡CCHClR 2 [R 2 =苯基,4-CF 3 PH,3- CNPH,4-(MEO 2 C)PH]在-50℃下导致形成的中间碳负离子(的ž)-1,2--C 6 H ^ 4 {C阿(= O)C≡C乙- [R 1 } {CH = CH(CH -)R 2 }(4)。低温(−50°C)有利于C B的侵蚀导致6,8个自行车的动力学形成,其中包含非经典的C-碳双烯酸烯醇化物(5)。较高的温度(相对于环境温度为−10°C)和缺乏电子的R 2有利于逆向σ键C-C裂解再生4,其随后在C A上关闭,提供6,6-双环醇盐(6)。计算模型(CBS-QB3)表明这两种途径都是可行的,并且具有相似的能量。6与H +反应生成1,2-二氢萘-1-醇,或在脱水条件下生成2-芳基-1-炔基萘。烯醇盐5与以下物质原位反应:H 2 O,D 2 O,I 2,烯丙基溴,S 2 Me 2,CO 2并直接从中间体5产生49–64%的预期C- E衍生物(E = H,D,I,烯丙基,SMe,CO 2 H)。母体(E = H; R 1 = SiMe 3,t Bu; R 2 = Ph)是用于NaBH 4和格利雅德C = O的添加,脱甲硅烷基化的通用原料(当R 1时)= SiMe)和肟的形成。后者允许通过贝克曼重排形成6,9-双环。6,8环碘化物是适合Pd催化的C-C偶联的Suzuki前体(81-87%),而羧酸在T3P®条件下很容易形成酰胺(71-95%)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号