当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyridine‐Catalysed Desulfonylative Addition of β‐Diketones to Arylazosulfones via Diaziridine Rearrangement
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-21 , DOI: 10.1002/adsc.202001171 Xin Ji 1 , Ling‐Guo Meng 1 , Hailong Xu 1 , Lei Wang 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-21 , DOI: 10.1002/adsc.202001171 Xin Ji 1 , Ling‐Guo Meng 1 , Hailong Xu 1 , Lei Wang 1, 2
Affiliation
|
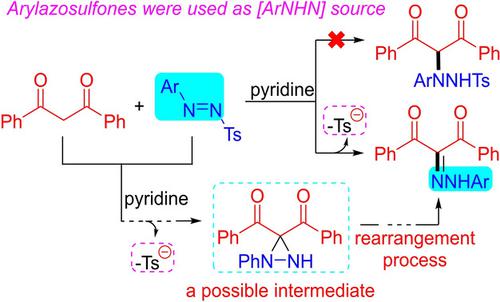
A pyridine‐catalysed desulfonylative addition of β‐diketones to arylazosulfones was developed to obtain diazenyl β‐dicarbonyl compounds. The aryldiazenyl group was observed in the desired product from arylazosulfones, and this diazenylation reaction was achieved via a possible rearrangement process based on diaziridine ring cleavage. The scope of the protocol was investigated and a plausible mechanism was given.
中文翻译:

吡啶催化的二氮丙啶重排将β-二酮脱磺酰基加成至芳偶氮砜
开发了吡啶催化的β-二酮向芳基偶氮砜的脱磺酰基加成反应,以获得二氮烯基β-二羰基化合物。在所需的芳基偶氮砜产物中观察到芳基二氮烯基,该二氮烯基化反应是通过基于二氮丙啶环裂解的可能的重排过程实现的。研究了协议的范围,并给出了合理的机制。
更新日期:2021-02-16
中文翻译:

吡啶催化的二氮丙啶重排将β-二酮脱磺酰基加成至芳偶氮砜
开发了吡啶催化的β-二酮向芳基偶氮砜的脱磺酰基加成反应,以获得二氮烯基β-二羰基化合物。在所需的芳基偶氮砜产物中观察到芳基二氮烯基,该二氮烯基化反应是通过基于二氮丙啶环裂解的可能的重排过程实现的。研究了协议的范围,并给出了合理的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号