当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine/DMSO-Promoted Selective Direct Arylthiation of Anilines with Thiols under Metal-Free Conditions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-21 , DOI: 10.1021/acs.joc.0c02078 Wenqi Zhao 1 , Feng Zhang 1, 2 , Guo-Jun Deng 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-21 , DOI: 10.1021/acs.joc.0c02078 Wenqi Zhao 1 , Feng Zhang 1, 2 , Guo-Jun Deng 1
Affiliation
|
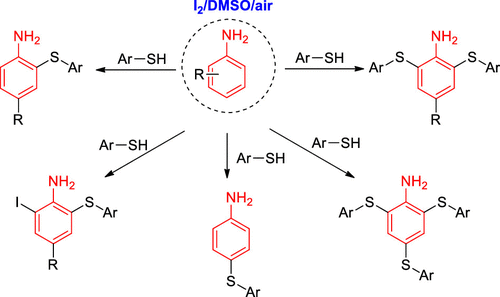
An iodine-promoted divergent thiolation of unprotected anilines with thiols for the synthesis of sulfide anilines has been described. The combinational use of I2 and DMSO played an important role to realize this kind of transformation without the aid of a metal catalyst and strong oxidants. The reaction selectivity was well controlled to provide mono-, bis-, and trisubstituted diaryl sulfide derivatives. More importantly, iodination and sulfenylation can occur simultaneously to provide useful multifunctionalized iodoaniline products. This method afforded an efficient protocol for the construct C–S and C–I bonds from the C–H bond under mild reaction conditions.
中文翻译:

无金属条件下碘/ DMSO促进的苯胺与硫醇的选择性直接芳基化
已经描述了碘促进的未保护苯胺与硫醇的发散硫醇化反应,用于合成硫化物苯胺。在不借助金属催化剂和强氧化剂的情况下,I 2和DMSO的组合使用对实现这种转化起了重要作用。很好地控制了反应的选择性,以提供单,双和三取代的二芳基硫醚衍生物。更重要的是,碘化和亚磺酰化可以同时发生,以提供有用的多官能化碘苯胺产物。该方法为在温和的反应条件下由C–H键构建C–S和C–I键提供了有效的方案。
更新日期:2021-01-01
中文翻译:

无金属条件下碘/ DMSO促进的苯胺与硫醇的选择性直接芳基化
已经描述了碘促进的未保护苯胺与硫醇的发散硫醇化反应,用于合成硫化物苯胺。在不借助金属催化剂和强氧化剂的情况下,I 2和DMSO的组合使用对实现这种转化起了重要作用。很好地控制了反应的选择性,以提供单,双和三取代的二芳基硫醚衍生物。更重要的是,碘化和亚磺酰化可以同时发生,以提供有用的多官能化碘苯胺产物。该方法为在温和的反应条件下由C–H键构建C–S和C–I键提供了有效的方案。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号