Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sialylation acts as a checkpoint for innate immune responses in the central nervous system
Glia ( IF 5.4 ) Pub Date : 2020-12-19 , DOI: 10.1002/glia.23945 Christine Klaus 1 , Huan Liao 1 , David H Allendorf 2 , Guy C Brown 2 , Harald Neumann 1
Glia ( IF 5.4 ) Pub Date : 2020-12-19 , DOI: 10.1002/glia.23945 Christine Klaus 1 , Huan Liao 1 , David H Allendorf 2 , Guy C Brown 2 , Harald Neumann 1
Affiliation
|
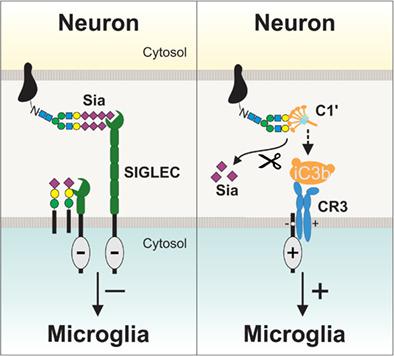
Sialic acids are monosaccharides that normally terminate the glycan chains of cell surface glyco‐proteins and ‐lipids in mammals, and are highly enriched in the central nervous tissue. Sialic acids are conjugated to proteins and lipids (termed “sialylation”) by specific sialyltransferases, and are removed (“desialylation”) by neuraminidases. Cell surface sialic acids are sensed by complement factor H (FH) to inhibit complement activation or by sialic acid‐binding immunoglobulin‐like lectin (SIGLEC) receptors to inhibit microglial activation, phagocytosis, and oxidative burst. In contrast, desialylation of cells enables binding of the opsonins C1, calreticulin, galectin‐3, and collectins, stimulating phagocytosis of such cells. Hypersialylation is used by bacteria and cancers as camouflage to escape immune recognition, while polysialylation of neurons protects synapses and neurogenesis. Insufficient lysosomal cleavage of sialylated molecules can lead to lysosomal accumulation of lipids and aggregated proteins, which if excessive may be expelled into the extracellular space. On the other hand, desialylation of immune receptors can activate them or trigger removal of proteins. Loss of inhibitory SIGLECs or FH triggers reduced clearance of aggregates, oxidative brain damage and complement‐mediated retinal damage. Thus, cell surface sialylation recognized by FH, SIGLEC, and other immune‐related receptors acts as a major checkpoint inhibitor of innate immune responses in the central nervous system, while excessive cleavage of sialic acid residues and consequently removing this checkpoint inhibitor may trigger lipid accumulation, protein aggregation, inflammation, and neurodegeneration.
中文翻译:

唾液酸化作为中枢神经系统先天免疫反应的检查点
唾液酸是通常终止哺乳动物细胞表面糖蛋白和脂质的聚糖链的单糖,在中枢神经组织中高度富集。唾液酸通过特定的唾液酸转移酶与蛋白质和脂质结合(称为“唾液酸化”),并通过神经氨酸酶去除(“去唾液酸化”)。细胞表面唾液酸由补体因子 H (FH) 感知以抑制补体激活,或由唾液酸结合免疫球蛋白样凝集素 (SIGLEC) 受体感知以抑制小胶质细胞激活、吞噬作用和氧化爆发。相比之下,细胞的去唾液酸化使调理素 C1、钙网蛋白、半乳凝素-3 和凝集素结合,刺激这些细胞的吞噬作用。过度唾液酸化被细菌和癌症用作逃避免疫识别的伪装,而神经元的多唾液酸化保护突触和神经发生。唾液酸化分子的溶酶体切割不足会导致脂质和聚集蛋白的溶酶体积累,如果过多可能会被排入细胞外空间。另一方面,免疫受体的去唾液酸化可以激活它们或触发蛋白质的去除。抑制性 SIGLEC 或 FH 的缺失会触发聚集体清除减少、氧化性脑损伤和补体介导的视网膜损伤。因此,被 FH、SIGLEC 和其他免疫相关受体识别的细胞表面唾液酸化可作为中枢神经系统先天免疫反应的主要检查点抑制剂,而唾液酸残基的过度切割并因此去除该检查点抑制剂可能引发脂质积累, 蛋白质聚集, 炎症,
更新日期:2020-12-19
中文翻译:

唾液酸化作为中枢神经系统先天免疫反应的检查点
唾液酸是通常终止哺乳动物细胞表面糖蛋白和脂质的聚糖链的单糖,在中枢神经组织中高度富集。唾液酸通过特定的唾液酸转移酶与蛋白质和脂质结合(称为“唾液酸化”),并通过神经氨酸酶去除(“去唾液酸化”)。细胞表面唾液酸由补体因子 H (FH) 感知以抑制补体激活,或由唾液酸结合免疫球蛋白样凝集素 (SIGLEC) 受体感知以抑制小胶质细胞激活、吞噬作用和氧化爆发。相比之下,细胞的去唾液酸化使调理素 C1、钙网蛋白、半乳凝素-3 和凝集素结合,刺激这些细胞的吞噬作用。过度唾液酸化被细菌和癌症用作逃避免疫识别的伪装,而神经元的多唾液酸化保护突触和神经发生。唾液酸化分子的溶酶体切割不足会导致脂质和聚集蛋白的溶酶体积累,如果过多可能会被排入细胞外空间。另一方面,免疫受体的去唾液酸化可以激活它们或触发蛋白质的去除。抑制性 SIGLEC 或 FH 的缺失会触发聚集体清除减少、氧化性脑损伤和补体介导的视网膜损伤。因此,被 FH、SIGLEC 和其他免疫相关受体识别的细胞表面唾液酸化可作为中枢神经系统先天免疫反应的主要检查点抑制剂,而唾液酸残基的过度切割并因此去除该检查点抑制剂可能引发脂质积累, 蛋白质聚集, 炎症,































 京公网安备 11010802027423号
京公网安备 11010802027423号