当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Oxygen Reduction to Hydrogen Peroxide: From Homogeneous to Heterogeneous Electrocatalysis
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-12-18 , DOI: 10.1002/aenm.202003323 Yulin Wang 1, 2 , Geoffrey I. N. Waterhouse 3 , Lu Shang 1, 2 , Tierui Zhang 1, 2
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-12-18 , DOI: 10.1002/aenm.202003323 Yulin Wang 1, 2 , Geoffrey I. N. Waterhouse 3 , Lu Shang 1, 2 , Tierui Zhang 1, 2
Affiliation
|
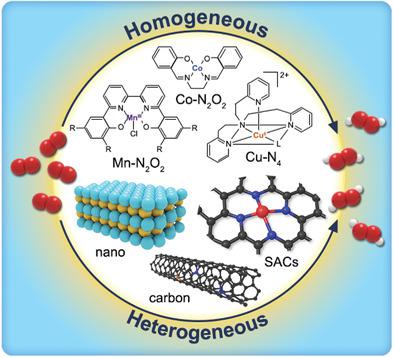
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant, finding widespread use across the chemical industry, in sanitation and environmental remediation. Currently, H2O2 is manufactured via the anthraquinone process which has a number of disadvantages including nondistributed production, high‐energy consumption, substantial organic by‐product waste, and the need to transport the obtained H2O2 to the point‐of‐use. Accordingly, the electrochemical synthesis of H2O2 is now attracting a lot of interest as an alternative, cost‐effective, small‐scale, and distributed technology for H2O2 manufacture. This review summarizes recent advancements in the development of Homogeneous and Heterogeneous catalysts for electrocatalytic O2 reduction reaction (ORR) to H2O2. The basic principles of the ORR, and methodologies for investigating the ORR to H2O2 are first introduced. Next, H2O2 production over Homogeneous catalysts is discussed, with a focus on the reaction mechanisms and the factors that influence activity, selectivity, and reaction kinetics. Subsequently, recent breakthroughs in H2O2 synthesis over Heterogeneous catalysts, including nonnoble metal‐based nanomaterials, carbon materials, and single‐atom catalysts are described. The latter are given special attention, since they serve as a bridge between Homogeneous catalysis and Heterogeneous catalysis, while also offering excellent performance. Finally, the challenges and opportunities for electrochemical ORR to H2O2 are critically discussed.
中文翻译:

电催化氧还原为过氧化氢:从均相到非均相电催化
过氧化氢(H 2 O 2)是一种环境友好的氧化剂,在整个化学工业中的卫生和环境修复中得到了广泛的应用。目前,H 2 O 2是通过蒽醌工艺制造的,存在许多缺点,包括分布不均,能耗高,有机副产物大量浪费以及需要将所获得的H 2 O 2运送到现场。 -用。因此,H的电化学合成2 ö 2现在吸引了很大兴趣作为替代,具有成本效益,小规模,并进行H分布式技术2 ö 2生产。这篇综述总结了用于电催化O 2还原反应(ORR)生成H 2 O 2的均相和非均相催化剂的最新进展。首先介绍了ORR的基本原理,以及研究ORR对H 2 O 2的方法。接下来,将讨论在均相催化剂上生产H 2 O 2,重点是反应机理和影响活性,选择性和反应动力学的因素。随后,H 2 O 2的最新突破描述了非均相催化剂(包括基于非贵金属的纳米材料,碳材料和单原子催化剂)上的合成。后者特别受关注,因为它们充当了均相催化和非均相催化之间的桥梁,同时还提供了出色的性能。最后,关键地讨论了电化学ORR制H 2 O 2的挑战和机遇。
更新日期:2020-12-18
中文翻译:

电催化氧还原为过氧化氢:从均相到非均相电催化
过氧化氢(H 2 O 2)是一种环境友好的氧化剂,在整个化学工业中的卫生和环境修复中得到了广泛的应用。目前,H 2 O 2是通过蒽醌工艺制造的,存在许多缺点,包括分布不均,能耗高,有机副产物大量浪费以及需要将所获得的H 2 O 2运送到现场。 -用。因此,H的电化学合成2 ö 2现在吸引了很大兴趣作为替代,具有成本效益,小规模,并进行H分布式技术2 ö 2生产。这篇综述总结了用于电催化O 2还原反应(ORR)生成H 2 O 2的均相和非均相催化剂的最新进展。首先介绍了ORR的基本原理,以及研究ORR对H 2 O 2的方法。接下来,将讨论在均相催化剂上生产H 2 O 2,重点是反应机理和影响活性,选择性和反应动力学的因素。随后,H 2 O 2的最新突破描述了非均相催化剂(包括基于非贵金属的纳米材料,碳材料和单原子催化剂)上的合成。后者特别受关注,因为它们充当了均相催化和非均相催化之间的桥梁,同时还提供了出色的性能。最后,关键地讨论了电化学ORR制H 2 O 2的挑战和机遇。

































 京公网安备 11010802027423号
京公网安备 11010802027423号