当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ions Slow Water Dynamics at Nonionic Surfactant Interfaces
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.jpcb.0c09086 Christopher P. Baryiames 1 , Emily Ma 2 , Carlos R. Baiz 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.jpcb.0c09086 Christopher P. Baryiames 1 , Emily Ma 2 , Carlos R. Baiz 1
Affiliation
|
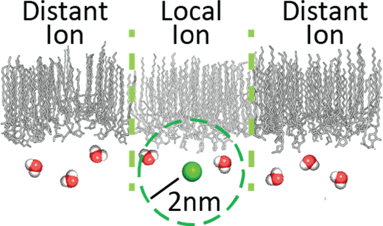
Nonionic surfactant interfaces are often considered to be unaffected by aqueous ions. However, ions often localize to interfaces and, as such, can interact with surfactants either via direct contact or by affecting interfacial hydrogen bond structures in water. Characterizing these effects is essential to understanding how ions affect interfacial properties at the oil–water interface in the presence of nonionic surfactants. Here, we use two-dimensional infrared (2D IR) spectroscopy in combination with atomistic molecular dynamics (MD) simulations to study the effects of high-concentration Na+ and Ca2+ ions on interfacial hydrogen bond dynamics in heterogeneous sorbitan stearate reverse micelles. Experiments show only minor changes in interfacial hydrogen bond populations when salts are added but those interfacial water network dynamics are slowed by nearly 300%. Molecular dynamics simulations show the slowdown results from an increased disorder in surfactant headgroup orientation and packing density, which stabilizes hydrogen bonding interactions between surfactants and interfacial water.
中文翻译:

非离子表面活性剂界面上的离子慢水动力学
通常认为非离子表面活性剂界面不受水性离子的影响。但是,离子通常位于界面处,因此可以通过直接接触或通过影响水中的界面氢键结构而与表面活性剂相互作用。表征这些效应对于理解离子在非离子表面活性剂存在下如何影响油水界面的界面特性至关重要。在这里,我们使用二维红外(2D IR)光谱结合原子分子动力学(MD)模拟来研究高浓度Na +和Ca 2+的影响离子对异质脱水山梨糖醇硬脂酸酯反胶束中界面氢键动力学的影响。实验表明,当添加盐时,界面氢键的数量只有很小的变化,但是界面水网络的动力学却减慢了近300%。分子动力学模拟显示,表面活性剂头基取向和堆积密度增加,从而稳定了表面活性剂与界面水之间的氢键相互作用,从而减慢了结果。
更新日期:2020-12-31
中文翻译:

非离子表面活性剂界面上的离子慢水动力学
通常认为非离子表面活性剂界面不受水性离子的影响。但是,离子通常位于界面处,因此可以通过直接接触或通过影响水中的界面氢键结构而与表面活性剂相互作用。表征这些效应对于理解离子在非离子表面活性剂存在下如何影响油水界面的界面特性至关重要。在这里,我们使用二维红外(2D IR)光谱结合原子分子动力学(MD)模拟来研究高浓度Na +和Ca 2+的影响离子对异质脱水山梨糖醇硬脂酸酯反胶束中界面氢键动力学的影响。实验表明,当添加盐时,界面氢键的数量只有很小的变化,但是界面水网络的动力学却减慢了近300%。分子动力学模拟显示,表面活性剂头基取向和堆积密度增加,从而稳定了表面活性剂与界面水之间的氢键相互作用,从而减慢了结果。





























 京公网安备 11010802027423号
京公网安备 11010802027423号