当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-metal-free synthesis of thiazolidin-2-ones and 1,3-thiazinan-2-ones from arylamines, elemental sulfur and CO2
Green Chemistry ( IF 9.3 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0gc03723k
Chuan-Kun Ran 1, 2, 3, 4, 5 , Lei Song 1, 2, 3, 4, 5 , Ya-Nan Niu 1, 2, 3, 4, 5 , Ming-Kai Wei 1, 2, 3, 4, 5 , Zhen Zhang 5, 6, 7, 8, 9 , Xiao-Yu Zhou 1, 2, 3, 4, 5 , Da-Gang Yu 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0gc03723k
Chuan-Kun Ran 1, 2, 3, 4, 5 , Lei Song 1, 2, 3, 4, 5 , Ya-Nan Niu 1, 2, 3, 4, 5 , Ming-Kai Wei 1, 2, 3, 4, 5 , Zhen Zhang 5, 6, 7, 8, 9 , Xiao-Yu Zhou 1, 2, 3, 4, 5 , Da-Gang Yu 1, 2, 3, 4, 5
Affiliation
|
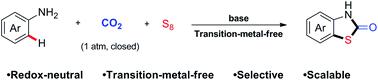
Transfering waste to treasure is highly important in green chemistry. However, it is difficult to realize it efficiently due to the low reactivity, especially the simultaneous utilization of two unreactive feedstocks in one reaction. Herein, we report the first utilization of both elemental sulfur and CO2 in a multi-component reaction to generate valuable thiazolidin-2-ones and 1,3-thiazinan-2-ones. Under transition-metal-free reaction conditions, a variety of easily available arylamines react with elemental sulfur and CO2 (1 atm) to give functional thiazolidin-2-ones and 1,3-thiazinan-2-ones in moderate to good yields via C–H bond functionalization. This strategy is highlighted by high step economy with generation of three bonds in one reaction and good functional groups tolerance.
中文翻译:

由芳基胺,元素硫和CO2合成无过渡金属的噻唑烷二酮和1,3-噻嗪南-2-酮
在绿色化学中,将废物转化为珍宝非常重要。但是,由于反应性低,特别是在一个反应中同时利用两种非反应性原料,因此难以有效地实现。在此,我们报告了在多组分反应中首次利用元素硫和CO 2生成有价值的噻唑烷二-2-酮和1,3-噻嗪南-2-酮。下过渡金属无反应条件,多种容易获得的芳基胺的与元素硫和CO反应2(1大气压)至以良好的产率得到官能噻唑烷-2-酮和1,3-噻嗪烷-2-酮在中度经由C–H键功能化。该策略的突出特点是高步经济性,可在一个反应中生成三个键并具有良好的官能团耐受性。
更新日期:2020-12-16
中文翻译:

由芳基胺,元素硫和CO2合成无过渡金属的噻唑烷二酮和1,3-噻嗪南-2-酮
在绿色化学中,将废物转化为珍宝非常重要。但是,由于反应性低,特别是在一个反应中同时利用两种非反应性原料,因此难以有效地实现。在此,我们报告了在多组分反应中首次利用元素硫和CO 2生成有价值的噻唑烷二-2-酮和1,3-噻嗪南-2-酮。下过渡金属无反应条件,多种容易获得的芳基胺的与元素硫和CO反应2(1大气压)至以良好的产率得到官能噻唑烷-2-酮和1,3-噻嗪烷-2-酮在中度经由C–H键功能化。该策略的突出特点是高步经济性,可在一个反应中生成三个键并具有良好的官能团耐受性。































 京公网安备 11010802027423号
京公网安备 11010802027423号