当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substituted 2,4-Di(pyridin-2-yl)pyrimidine-Based Ruthenium Photosensitizers for Hydrogen Photoevolution under Red Light
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-12-15 , DOI: 10.1021/acs.inorgchem.0c02955 Mira T. Rupp 1, 2 , Thomas Auvray 1 , Natali Shevchenko 1 , Lukas Swoboda 2 , Garry S. Hanan 1 , Dirk G. Kurth 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-12-15 , DOI: 10.1021/acs.inorgchem.0c02955 Mira T. Rupp 1, 2 , Thomas Auvray 1 , Natali Shevchenko 1 , Lukas Swoboda 2 , Garry S. Hanan 1 , Dirk G. Kurth 2
Affiliation
|
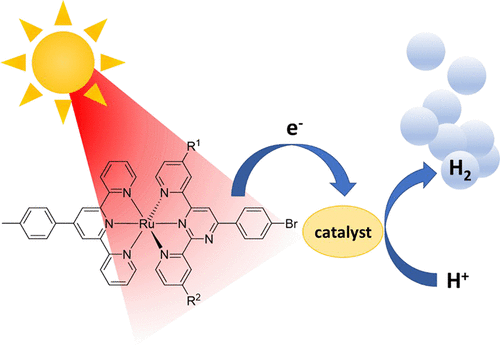
The photocatalytic reduction of water to form hydrogen gas (H2) is a promising approach to collect, convert, and store solar energy. Typically, ruthenium tris(bipyridine) and its many derivatives are used as photosensitizers (PSs) in a variety of photocatalytic conditions. The bis(terpyridine) analogues, however, have only recently gained attention for this application because of their poor photophysical properties. Yet, by the introduction of electron-donating or -withdrawing groups on the terpyridine ligands, the photophysical and electrochemical properties can be considerably improved. In this study, we report a series of nonsymmetric 2,6-di(pyridin-2-yl)pyrimidine ligands with peripheral pyridine substituents in different positions and their corresponding ruthenium(II) complexes. The presence of the pyrimidine ring stabilizes the lowest unoccupied molecular orbital, leading to a red-shifted emission and prolonged excited-state lifetimes as well as higher luminescence quantum yields compared to analogous terpyridine complexes. Furthermore, all complexes are easier to reduce than the previously reported bis(terpyridine) complexes used as PSs. Interestingly, the pyridine substituent in the 4-pyrimidine position has a greater impact on both the photophysical and electrochemical properties. This correlation between the substitution pattern and properties of the complexes is further investigated by using time-dependent density functional theory. In hydrogen evolution experiments under blue- and red-light irradiation, all investigated complexes exhibit much higher activity compared to the previously reported ruthenium(II) bis(terpyridine) complexes, but none of the complexes are as stable as the literature compounds, presumably because of an additional decomposition pathway of the reduced PS competing with electron transfer from the reduced PS to the catalyst.
中文翻译:

取代的2,4-二(吡啶-2-基)嘧啶基钌光敏剂用于红光下的氢光进化
水的光催化还原形成氢气(H 2)是一种收集,转换和储存太阳能的有前途的方法。通常,三(联吡啶)钌及其许多衍生物在多种光催化条件下用作光敏剂(PSs)。然而,双(叔吡啶)类似物由于其较差的光物理性质而在最近才引起关注。然而,通过在叔吡啶配体上引入给电子或吸电子基团,可以显着改善光物理和电化学性能。在这项研究中,我们报告了一系列不对称的2,6-二(吡啶-2-基)嘧啶配体,它们在不同位置具有周边的吡啶取代基及其相应的钌(II)配合物。嘧啶环的存在可稳定最低的未占据分子轨道,与类似的三联吡啶配合物相比,可导致红移发射和延长的激发态寿命,以及更高的发光量子产率。此外,所有复合物都比以前报道的用作PS的双(三联吡啶)复合物更容易还原。有趣的是,在4-嘧啶位置的吡啶取代基对光物理和电化学性质都具有更大的影响。通过使用时间依赖的密度泛函理论进一步研究了取代模式与配合物性质之间的这种相关性。在蓝光和红光辐射下的氢气析出实验中,所有研究的配合物均比以前报道的钌(II)双(叔吡啶)配合物具有更高的活性,
更新日期:2021-01-04
中文翻译:

取代的2,4-二(吡啶-2-基)嘧啶基钌光敏剂用于红光下的氢光进化
水的光催化还原形成氢气(H 2)是一种收集,转换和储存太阳能的有前途的方法。通常,三(联吡啶)钌及其许多衍生物在多种光催化条件下用作光敏剂(PSs)。然而,双(叔吡啶)类似物由于其较差的光物理性质而在最近才引起关注。然而,通过在叔吡啶配体上引入给电子或吸电子基团,可以显着改善光物理和电化学性能。在这项研究中,我们报告了一系列不对称的2,6-二(吡啶-2-基)嘧啶配体,它们在不同位置具有周边的吡啶取代基及其相应的钌(II)配合物。嘧啶环的存在可稳定最低的未占据分子轨道,与类似的三联吡啶配合物相比,可导致红移发射和延长的激发态寿命,以及更高的发光量子产率。此外,所有复合物都比以前报道的用作PS的双(三联吡啶)复合物更容易还原。有趣的是,在4-嘧啶位置的吡啶取代基对光物理和电化学性质都具有更大的影响。通过使用时间依赖的密度泛函理论进一步研究了取代模式与配合物性质之间的这种相关性。在蓝光和红光辐射下的氢气析出实验中,所有研究的配合物均比以前报道的钌(II)双(叔吡啶)配合物具有更高的活性,















































 京公网安备 11010802027423号
京公网安备 11010802027423号