当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of a Split Flavin-Based Fluorescent Reporter
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-12-16 , DOI: 10.1021/acssynbio.0c00454 Anna Yudenko 1 , Anastasia Smolentseva 1 , Ivan Maslov 1 , Oleg Semenov 1 , Ivan M Goncharov 1 , Vera V Nazarenko 1 , Nina L Maliar 1 , Valentin Borshchevskiy 1 , Valentin Gordeliy 1, 2, 3, 4 , Alina Remeeva 1 , Ivan Gushchin 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-12-16 , DOI: 10.1021/acssynbio.0c00454 Anna Yudenko 1 , Anastasia Smolentseva 1 , Ivan Maslov 1 , Oleg Semenov 1 , Ivan M Goncharov 1 , Vera V Nazarenko 1 , Nina L Maliar 1 , Valentin Borshchevskiy 1 , Valentin Gordeliy 1, 2, 3, 4 , Alina Remeeva 1 , Ivan Gushchin 1
Affiliation
|
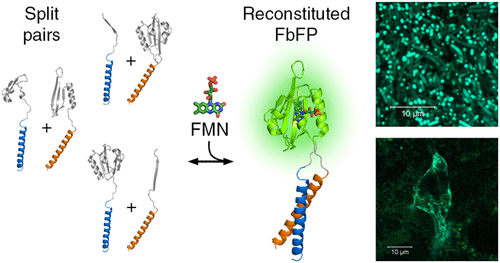
Protein-fragment complementation assays are used ubiquitously for probing protein–protein interactions. Most commonly, the reporter protein is split in two parts, which are then fused to the proteins of interest and can reassemble and provide a readout if the proteins of interest interact with each other. The currently known split fluorescent proteins either can be used only in aerobic conditions and assemble irreversibly, or require addition of exogenous chromophores, which complicates the design of experiments. In recent years, light-oxygen-voltage (LOV) domains of several photoreceptor proteins have been developed into flavin-based fluorescent proteins (FbFPs) that, under some circumstances, can outperform commonly used fluorescent proteins such as GFP. Here, we show that CagFbFP, a small thermostable FbFP based on a LOV domain-containing protein from Chloroflexus aggregans, can serve as a split fluorescent reporter. We use the available genetic and structural information to identify three loops between the conserved secondary structure elements, Aβ-Bβ, Eα-Fα, and Hβ-Iβ, that tolerate insertion of flexible poly-Gly/Ser segments and eventually splitting. We demonstrate that the designed split pairs, when fused to interacting proteins, are fluorescent in vivo in E. coli and human cells and have low background fluorescence. Our results enable probing protein–protein interactions in anaerobic conditions without using exogenous fluorophores and provide a basis for further development of LOV and PAS (Per-Arnt-Sim) domain-based fluorescent reporters and optogenetic tools.
中文翻译:

基于分裂黄素的荧光报告基因的合理设计
蛋白质片段互补分析普遍用于探测蛋白质-蛋白质相互作用。最常见的是,报告蛋白被分成两部分,然后与感兴趣的蛋白质融合,如果感兴趣的蛋白质相互作用,可以重新组装并提供读数。目前已知的分裂荧光蛋白要么只能在有氧条件下使用并且不可逆地组装,要么需要添加外源发色团,这使实验设计复杂化。近年来,几种光感受器蛋白的光氧电压 (LOV) 域已发展为基于黄素的荧光蛋白 (FbFP),在某些情况下,其性能优于常用的荧光蛋白,如 GFP。在这里,我们展示了 CagFbFP,Chloroflexus aggregans可作为分裂荧光报告基因。我们使用可用的遗传和结构信息来确定保守的二级结构元件 Aβ-Bβ、Eα-Fα 和 Hβ-Iβ 之间的三个环,这些环允许插入灵活的聚 Gly/Ser 片段并最终分裂。我们证明了设计的分裂对,当与相互作用的蛋白质融合时,在大肠杆菌和人类细胞中在体内发出荧光,并且具有低背景荧光。我们的结果能够在不使用外源荧光团的情况下探测厌氧条件下的蛋白质-蛋白质相互作用,并为进一步开发基于 LOV 和 PAS(Per-Arnt-Sim)域的荧光报告基因和光遗传学工具提供基础。
更新日期:2021-01-15
中文翻译:

基于分裂黄素的荧光报告基因的合理设计
蛋白质片段互补分析普遍用于探测蛋白质-蛋白质相互作用。最常见的是,报告蛋白被分成两部分,然后与感兴趣的蛋白质融合,如果感兴趣的蛋白质相互作用,可以重新组装并提供读数。目前已知的分裂荧光蛋白要么只能在有氧条件下使用并且不可逆地组装,要么需要添加外源发色团,这使实验设计复杂化。近年来,几种光感受器蛋白的光氧电压 (LOV) 域已发展为基于黄素的荧光蛋白 (FbFP),在某些情况下,其性能优于常用的荧光蛋白,如 GFP。在这里,我们展示了 CagFbFP,Chloroflexus aggregans可作为分裂荧光报告基因。我们使用可用的遗传和结构信息来确定保守的二级结构元件 Aβ-Bβ、Eα-Fα 和 Hβ-Iβ 之间的三个环,这些环允许插入灵活的聚 Gly/Ser 片段并最终分裂。我们证明了设计的分裂对,当与相互作用的蛋白质融合时,在大肠杆菌和人类细胞中在体内发出荧光,并且具有低背景荧光。我们的结果能够在不使用外源荧光团的情况下探测厌氧条件下的蛋白质-蛋白质相互作用,并为进一步开发基于 LOV 和 PAS(Per-Arnt-Sim)域的荧光报告基因和光遗传学工具提供基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号