Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling Heterogeneity of Triple‐Negative Breast Cancer Uncovers a Novel Combinatorial Treatment Overcoming Primary Drug Resistance
Advanced Science ( IF 14.3 ) Pub Date : 2020-12-16 , DOI: 10.1002/advs.202003049 Fabienne Lamballe 1 , Fahmida Ahmad 1 , Yaron Vinik 2 , Olivier Castellanet 1 , Fabrice Daian 1 , Anna-Katharina Müller 2 , Ulrike A Köhler 2 , Anne-Laure Bailly 3 , Emmanuelle Josselin 4 , Rémy Castellano 4 , Christelle Cayrou 3 , Emmanuelle Charafe-Jauffret 5 , Gordon B Mills 6 , Vincent Géli 3 , Jean-Paul Borg 3, 7 , Sima Lev 2 , Flavio Maina 1
Advanced Science ( IF 14.3 ) Pub Date : 2020-12-16 , DOI: 10.1002/advs.202003049 Fabienne Lamballe 1 , Fahmida Ahmad 1 , Yaron Vinik 2 , Olivier Castellanet 1 , Fabrice Daian 1 , Anna-Katharina Müller 2 , Ulrike A Köhler 2 , Anne-Laure Bailly 3 , Emmanuelle Josselin 4 , Rémy Castellano 4 , Christelle Cayrou 3 , Emmanuelle Charafe-Jauffret 5 , Gordon B Mills 6 , Vincent Géli 3 , Jean-Paul Borg 3, 7 , Sima Lev 2 , Flavio Maina 1
Affiliation
|
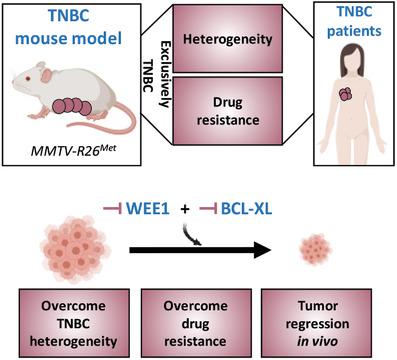
Triple‐negative breast cancer (TNBC) is a highly aggressive breast cancer subtype characterized by a remarkable molecular heterogeneity. Currently, there are no effective druggable targets and advanced preclinical models of the human disease. Here, a unique mouse model (MMTV‐R26Met mice) of mammary tumors driven by a subtle increase in the expression of the wild‐type MET receptor is generated. MMTV‐R26Met mice develop spontaneous, exclusive TNBC tumors, recapitulating primary resistance to treatment of patients. Proteomic profiling of MMTV‐R26Met tumors and machine learning approach show that the model faithfully recapitulates intertumoral heterogeneity of human TNBC. Further signaling network analysis highlights potential druggable targets, of which cotargeting of WEE1 and BCL‐XL synergistically kills TNBC cells and efficiently induces tumor regression. Mechanistically, BCL‐XL inhibition exacerbates the dependency of TNBC cells on WEE1 function, leading to Histone H3 and phosphoS33RPA32 upregulation, RRM2 downregulation, cell cycle perturbation, mitotic catastrophe, and apoptosis. This study introduces a unique, powerful mouse model for studying TNBC formation and evolution, its heterogeneity, and for identifying efficient therapeutic targets.
中文翻译:

三阴性乳腺癌的异质性建模揭示了一种克服原发性耐药性的新型组合疗法
三阴性乳腺癌(TNBC)是一种高度侵袭性的乳腺癌亚型,具有显着的分子异质性。目前,人类疾病尚无有效的药物靶点和先进的临床前模型。在这里,产生了一种独特的乳腺肿瘤小鼠模型( MMTV-R26 Met小鼠),该模型由野生型 MET 受体表达的微妙增加驱动。 MMTV-R26 Met小鼠产生自发的、独特的 TNBC 肿瘤,再现了患者对治疗的主要耐药性。 MMTV-R26 Met肿瘤的蛋白质组学分析和机器学习方法表明,该模型忠实地再现了人类 TNBC 的肿瘤间异质性。进一步的信号网络分析突出了潜在的药物靶点,其中 WEE1 和 BCL-XL 的共同靶向可协同杀死 TNBC 细胞并有效诱导肿瘤消退。从机制上讲,BCL-XL 抑制加剧了 TNBC 细胞对 WEE1 功能的依赖性,导致组蛋白 H3 和磷酸化 S 33 RPA32 上调、RRM2 下调、细胞周期扰动、有丝分裂灾难和细胞凋亡。这项研究引入了一种独特、强大的小鼠模型,用于研究 TNBC 的形成和进化、其异质性,并确定有效的治疗靶点。
更新日期:2021-02-03
中文翻译:

三阴性乳腺癌的异质性建模揭示了一种克服原发性耐药性的新型组合疗法
三阴性乳腺癌(TNBC)是一种高度侵袭性的乳腺癌亚型,具有显着的分子异质性。目前,人类疾病尚无有效的药物靶点和先进的临床前模型。在这里,产生了一种独特的乳腺肿瘤小鼠模型( MMTV-R26 Met小鼠),该模型由野生型 MET 受体表达的微妙增加驱动。 MMTV-R26 Met小鼠产生自发的、独特的 TNBC 肿瘤,再现了患者对治疗的主要耐药性。 MMTV-R26 Met肿瘤的蛋白质组学分析和机器学习方法表明,该模型忠实地再现了人类 TNBC 的肿瘤间异质性。进一步的信号网络分析突出了潜在的药物靶点,其中 WEE1 和 BCL-XL 的共同靶向可协同杀死 TNBC 细胞并有效诱导肿瘤消退。从机制上讲,BCL-XL 抑制加剧了 TNBC 细胞对 WEE1 功能的依赖性,导致组蛋白 H3 和磷酸化 S 33 RPA32 上调、RRM2 下调、细胞周期扰动、有丝分裂灾难和细胞凋亡。这项研究引入了一种独特、强大的小鼠模型,用于研究 TNBC 的形成和进化、其异质性,并确定有效的治疗靶点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号