当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Superior Polyaniline Cathode Material with Enhanced Capacity for Ammonium Ion Storage
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-12-15 , DOI: 10.1021/acsaem.0c01791 Shelton Farai Kuchena 1 , Ying Wang 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-12-15 , DOI: 10.1021/acsaem.0c01791 Shelton Farai Kuchena 1 , Ying Wang 1
Affiliation
|
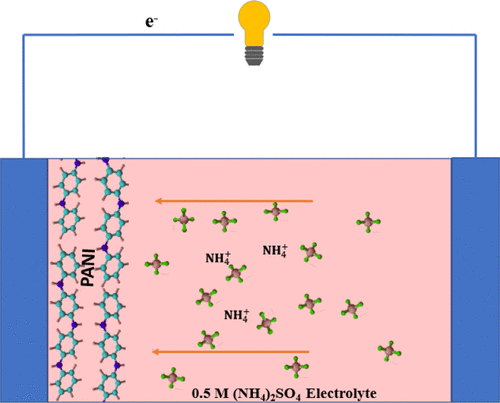
A paradigm shift in aqueous rechargeable batteries has seen the development of nonmetal ammonium ions (NH4+) as charge carriers. NH4+ ions have a molecular weight of 18 g mol–1 and hydrated ionic size of 3.31 Å, which are smaller than those of metal ions, leading to faster kinetics during charge transfer. Yet few electrode materials have been studied for this type of battery. This work is the first effort to show that polyaniline (PANI) is an excellent material for NH4+ ion storage, and thus further proves that NH4+ ions can be used as effective charge carriers in aqueous ion battery systems. A facile solution method is used to prepare emeraldine salt polyaniline (ES-PANI) on the carbon felts (CFs) as the cathode material. The battery cell based on the ES-PANI cathode material shows a good discharge capacity of 160 mAh g–1 at a specific current of 1 A g–1. At 5 A g–1, it shows a good capacity retention of 82% after 100 cycles and also exhibits excellent rate capability. Furthermore, it is found that the ES-PANI/CFs washed with water deliver higher capacity than those washed with ethanol, because washing with ethanol causes the oligomers to dissolve in the solution and thus decreases the storage capacity of ES-PANI for NH4+ ion storage. The intercalation/deintercalation of NH4+ ion is shown to be highly reversible in the ES-PANI electrode doped with Cl- ions, due to the stable redox properties of nitrogen in ES-PANI polymer chains. As such, this work sheds new insight into the exploration of alternative electrode material for ammonium ion storage, which can lead to new electrochemical energy technology.
中文翻译:

具有增强的铵离子存储能力的优质聚苯胺阴极材料
水性可充电电池的范式转变已经看到非金属铵离子(NH 4 +)作为电荷载体的发展。NH 4 +离子的分子量为18 g mol –1,水合离子的大小为3.31Å,小于金属离子的大小,从而导致电荷转移过程中动力学更快。很少有人研究这种电池的电极材料。这项工作是首次证明聚苯胺(PANI)是用于NH 4 +离子存储的极好材料,从而进一步证明了NH 4 +离子可在水性离子电池系统中用作有效的电荷载体。一种简便的溶液方法用于在作为阴极材料的碳毡(CFs)上制备翡翠盐聚苯胺(ES-PANI)。基于ES-PANI正极材料的电池在1 A g –1的特定电流下显示出160 mAh g –1的良好放电容量。在5 A g –1时,它在100次循环后显示出82%的良好容量保持率,并且还具有出色的倍率能力。此外,发现用水洗涤的ES-PANI / CFs的容量比用乙醇洗涤的ES-PANI / CF的容量高,因为用乙醇洗涤会导致低聚物溶解在溶液中,从而降低ES-PANI对NH 4 +的储存能力。离子存储。的嵌入/脱嵌NH 4 +离子被示出为在ES-PANI电极掺杂了Cl的高度可逆-离子,由于在ES-PANI聚合物链上的氮的稳定的氧化还原性质。这样,这项工作为探索用于铵离子存储的替代电极材料提供了新的见识,这可以导致新的电化学能源技术。
更新日期:2020-12-28
中文翻译:

具有增强的铵离子存储能力的优质聚苯胺阴极材料
水性可充电电池的范式转变已经看到非金属铵离子(NH 4 +)作为电荷载体的发展。NH 4 +离子的分子量为18 g mol –1,水合离子的大小为3.31Å,小于金属离子的大小,从而导致电荷转移过程中动力学更快。很少有人研究这种电池的电极材料。这项工作是首次证明聚苯胺(PANI)是用于NH 4 +离子存储的极好材料,从而进一步证明了NH 4 +离子可在水性离子电池系统中用作有效的电荷载体。一种简便的溶液方法用于在作为阴极材料的碳毡(CFs)上制备翡翠盐聚苯胺(ES-PANI)。基于ES-PANI正极材料的电池在1 A g –1的特定电流下显示出160 mAh g –1的良好放电容量。在5 A g –1时,它在100次循环后显示出82%的良好容量保持率,并且还具有出色的倍率能力。此外,发现用水洗涤的ES-PANI / CFs的容量比用乙醇洗涤的ES-PANI / CF的容量高,因为用乙醇洗涤会导致低聚物溶解在溶液中,从而降低ES-PANI对NH 4 +的储存能力。离子存储。的嵌入/脱嵌NH 4 +离子被示出为在ES-PANI电极掺杂了Cl的高度可逆-离子,由于在ES-PANI聚合物链上的氮的稳定的氧化还原性质。这样,这项工作为探索用于铵离子存储的替代电极材料提供了新的见识,这可以导致新的电化学能源技术。






































 京公网安备 11010802027423号
京公网安备 11010802027423号