当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium catalyzed 8-aminoimidazo[1,2-a]pyridine (AIP) directed selective β-C(sp2)–H arylation
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ob02134b Biswajit Mondal 1, 2, 3, 4, 5 , Prasanjit Ghosh 3, 4, 5, 6 , MrinalKanti Kundu 1, 2, 3 , Tapas Kumar Das 1, 2, 3, 4, 5 , Sajal Das 3, 4, 5, 6
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ob02134b Biswajit Mondal 1, 2, 3, 4, 5 , Prasanjit Ghosh 3, 4, 5, 6 , MrinalKanti Kundu 1, 2, 3 , Tapas Kumar Das 1, 2, 3, 4, 5 , Sajal Das 3, 4, 5, 6
Affiliation
|
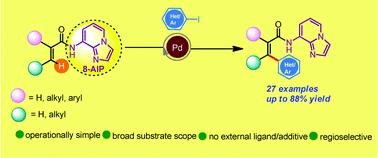
Palladium catalyzed arylation of the inert β-C(sp2)–H bond of carboxylic acid derivatives is reported herein for the first time utilizing 8-aminoimidazo[1,2-a]pyridine (AIP) as an efficacious and new inbuilt 6,5-fused bicyclic removable directing group. This protocol is scalable, exhibits high levels of β-site selectivity and tolerates a broad spectrum of functional groups.
中文翻译:

钯催化的8-氨基咪唑并[1,2-a]吡啶(AIP)定向选择性β-C(sp2)–H芳基化
本文首次报道了羧酸衍生物的惰性β-C(sp 2)-H键的钯催化的芳基化反应,首次使用8-氨基咪唑并[1,2- a ]吡啶(AIP)作为有效的新型内嵌化合物6, 5-稠合的双环可移动导向基团。该协议是可扩展的,具有高水平的β-位点选择性,并能耐受各种官能团。
更新日期:2020-12-15
中文翻译:

钯催化的8-氨基咪唑并[1,2-a]吡啶(AIP)定向选择性β-C(sp2)–H芳基化
本文首次报道了羧酸衍生物的惰性β-C(sp 2)-H键的钯催化的芳基化反应,首次使用8-氨基咪唑并[1,2- a ]吡啶(AIP)作为有效的新型内嵌化合物6, 5-稠合的双环可移动导向基团。该协议是可扩展的,具有高水平的β-位点选择性,并能耐受各种官能团。































 京公网安备 11010802027423号
京公网安备 11010802027423号