当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of cobalt oxyhydroxide nanosheets with rich oxygen vacancies as high-performance lithium-ion battery anodes
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ta10389f Yonghuan Fu 1, 2, 3, 4, 5 , Liewu Li 1, 2, 3, 4, 5 , Shenghua Ye 1, 2, 3, 4, 5 , Penggang Yang 1, 2, 3, 4, 5 , Peng Liao 6, 7, 8, 9 , Xiangzhong Ren 1, 2, 3, 4, 5 , Chuanxin He 1, 2, 3, 4, 5 , Qianling Zhang 1, 2, 3, 4, 5 , Jianhong Liu 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ta10389f Yonghuan Fu 1, 2, 3, 4, 5 , Liewu Li 1, 2, 3, 4, 5 , Shenghua Ye 1, 2, 3, 4, 5 , Penggang Yang 1, 2, 3, 4, 5 , Peng Liao 6, 7, 8, 9 , Xiangzhong Ren 1, 2, 3, 4, 5 , Chuanxin He 1, 2, 3, 4, 5 , Qianling Zhang 1, 2, 3, 4, 5 , Jianhong Liu 1, 2, 3, 4, 5
Affiliation
|
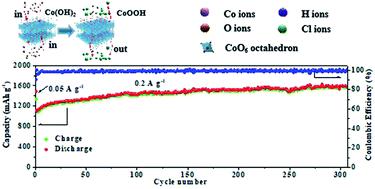
Cobalt oxyhydroxide (CoOOH) is a promising anode material for lithium-ion batteries (LIBs) due to its high electronic conductivity (5 S cm−1) and theoretical specific capacity (1457 mA h g−1). Herein, CoOOH nanosheets are successfully obtained using a facile one-pot method, and a hierarchical nanoporous structure is formed by oxidizing cobalt hydroxide (Co(OH)2) in NaOH and (NH4)2S2O8 solution. The CoOOH anode shows better electrochemical performance compared to Co(OH)2 and Co3O4 electrodes when applied to LIBs. The hierarchical nanoporous structure and high electronic conductivity of the CoOOH anode contribute to its outstanding initial discharge capacity (1478 mA h g−1 at 0.2 A g−1), high initial coulombic efficiency (ICE, 90%), and excellent cyclability (1588 mA h g−1 after 300 cycles). Experiments and density functional theory (DFT) calculations confirmed that the high ICE and prominent rate capability (574 mA h g−1 at 5 A g−1) of the nanosheets could be ascribed to the rapid and complete conversion reaction of CoOOH upon lithiation/delithiation facilitated by hydroxyl groups and oxygen vacancies. This study provides new insights into the structure–property relationship of transition-metal oxyhydroxide anode materials for LIBs.
中文翻译:

具有丰富氧空位的羟基氧化钴纳米片的构建,作为高性能锂离子电池阳极
羟基氧化钴(CoOOH)由于其高电导率(5 S cm -1)和理论比容量(1457 mA hg -1)而成为锂离子电池(LIB)的有希望的负极材料。本文中,使用简便的一锅法成功地获得了CoOOH纳米片,并且通过在NaOH和(NH 4)2 S 2 O 8溶液中氧化氢氧化钴(Co(OH)2)形成了分层的纳米孔结构。与Co(OH)2和Co 3 O 4相比,CoOOH阳极表现出更好的电化学性能电极应用于LIB时。的CoOOH的阳极的分层纳米多孔结构和高的电子传导性有助于其优异的初始放电容量(1478毫安汞柱-1 0.2 A G -1),高的初始库仑效率(ICE,90%),以及优良的可循环性(1588毫安300次循环后,hg -1)。实验和密度泛函理论(DFT)计算证实,高ICE和突出速率能力(574毫安汞柱-1在5 A G -1)的纳米片可以归因于通过羟基和氧空位促进的锂化/脱锂时CoOOH的快速和完全转化反应。这项研究为LIB的过渡金属羟基氧化物阳极材料的结构与性质之间的关系提供了新的见解。
更新日期:2020-12-15
中文翻译:

具有丰富氧空位的羟基氧化钴纳米片的构建,作为高性能锂离子电池阳极
羟基氧化钴(CoOOH)由于其高电导率(5 S cm -1)和理论比容量(1457 mA hg -1)而成为锂离子电池(LIB)的有希望的负极材料。本文中,使用简便的一锅法成功地获得了CoOOH纳米片,并且通过在NaOH和(NH 4)2 S 2 O 8溶液中氧化氢氧化钴(Co(OH)2)形成了分层的纳米孔结构。与Co(OH)2和Co 3 O 4相比,CoOOH阳极表现出更好的电化学性能电极应用于LIB时。的CoOOH的阳极的分层纳米多孔结构和高的电子传导性有助于其优异的初始放电容量(1478毫安汞柱-1 0.2 A G -1),高的初始库仑效率(ICE,90%),以及优良的可循环性(1588毫安300次循环后,hg -1)。实验和密度泛函理论(DFT)计算证实,高ICE和突出速率能力(574毫安汞柱-1在5 A G -1)的纳米片可以归因于通过羟基和氧空位促进的锂化/脱锂时CoOOH的快速和完全转化反应。这项研究为LIB的过渡金属羟基氧化物阳极材料的结构与性质之间的关系提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号