当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling Hydrogen Evolution Reaction Kinetics through Explicit Water–Metal Interfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-15 , DOI: 10.1021/acs.jpcc.0c08310 Michael T. Tang 1, 2 , Xinyan Liu 2 , Yongfei Ji 3 , Jens K. Norskov 4 , Karen Chan 4
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-15 , DOI: 10.1021/acs.jpcc.0c08310 Michael T. Tang 1, 2 , Xinyan Liu 2 , Yongfei Ji 3 , Jens K. Norskov 4 , Karen Chan 4
Affiliation
|
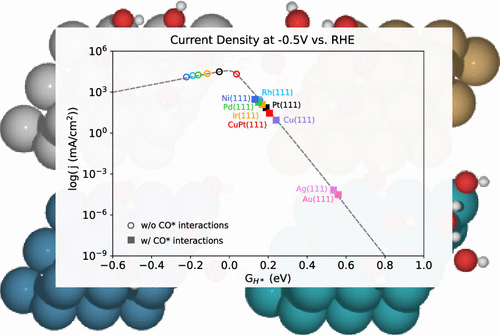
Despite the apparent simplicity of the hydrogen evolution reaction (HER) and the decades of research into it, controversy remains in the literature regarding the identity of the active site and the competition between the Heyrovsky and Tafel steps. In this work, we use charge-extrapolated ab initio simulations with explicit water in conjunction with mean-field microkinetic modeling to explore the mechanism for HER on both close-packed (111) and stepped (211) transition metals. First, we show that atop H*, beyond a monolayer of hollow H*, is unlikely to play a role in the HER mechanism, given its very positive adsorption energies. The energetics suggests the Volmer–Heyrovsky mechanism to predominate on fcc transition metals under typical operating conditions. We evaluate our theoretical results vs several experimental observations. We show that the Volmer–Heyrovsky mechanism predicts an activity volcano with its peak at a H* binding ΔGH* ≈ 0 eV, consistent with experiment. In contrast, the Volmer–Tafel volcano shows a broad rate plateau between ΔGH* ≈ 0 eV and ΔGH* ≈ – 0.4 eV. We find our theoretical Tafel slopes to be consistent with experimental ones on a range of transition metals. We show that, in line with experimental observations, the introduction of a CO(g) atmosphere shifts the strong binding metals toward the weak binding leg. Our study suggests that the simple thermodynamic approach to HER activity still holds, even when a detailed kinetic picture is considered.
中文翻译:

通过明确的水-金属界面模拟氢释放反应动力学
尽管氢放出反应(HER)的表面看起来很简单,并且进行了数十年的研究,但关于活性位点的身份以及Heyrovsky和Tafel步骤之间的竞争的争论仍存在于文献中。在这项工作中,我们将电荷与显性水一起使用,从头算模拟与平均场微动力学模型一起使用,以探索密排(111)和阶梯(211)过渡金属上HER的机理。首先,我们表明,由于空心H *的单层非常强的吸附能,其上方的H *不可能超出HER机理。能量学表明,在典型的操作条件下,Volmer-Heyrovsky机理在fcc过渡金属中占主导地位。我们通过几个实验观察来评估我们的理论结果。G H * ≈0 eV,与实验一致。相反,沃尔默-塔菲尔火山在ΔG H * ≈0 eV和ΔG H * ≈– 0.4 eV之间显示出较宽的速率平台。我们发现我们的理论塔菲尔斜率与一系列过渡金属上的实验斜率一致。我们表明,与实验观察结果一致,CO(g)气氛的引入将强结合金属移向弱结合腿。我们的研究表明,即使考虑到详细的动力学图谱,仍然可以采用简单的热力学方法来研究HER活性。
更新日期:2020-12-24
中文翻译:

通过明确的水-金属界面模拟氢释放反应动力学
尽管氢放出反应(HER)的表面看起来很简单,并且进行了数十年的研究,但关于活性位点的身份以及Heyrovsky和Tafel步骤之间的竞争的争论仍存在于文献中。在这项工作中,我们将电荷与显性水一起使用,从头算模拟与平均场微动力学模型一起使用,以探索密排(111)和阶梯(211)过渡金属上HER的机理。首先,我们表明,由于空心H *的单层非常强的吸附能,其上方的H *不可能超出HER机理。能量学表明,在典型的操作条件下,Volmer-Heyrovsky机理在fcc过渡金属中占主导地位。我们通过几个实验观察来评估我们的理论结果。G H * ≈0 eV,与实验一致。相反,沃尔默-塔菲尔火山在ΔG H * ≈0 eV和ΔG H * ≈– 0.4 eV之间显示出较宽的速率平台。我们发现我们的理论塔菲尔斜率与一系列过渡金属上的实验斜率一致。我们表明,与实验观察结果一致,CO(g)气氛的引入将强结合金属移向弱结合腿。我们的研究表明,即使考虑到详细的动力学图谱,仍然可以采用简单的热力学方法来研究HER活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号