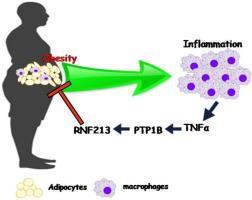
糖尿病患者总是处于缺血性疾病如冠状动脉疾病的较高风险中。一种这样的缺血性颈动脉疾病是与I型和II型糖尿病有关的烟雾病(MMD),但因果关系尚不清楚。无名指蛋白213(RNF213)是MMD的主要易感基因。为了了解糖尿病和MMD之间的关系,我们从两个异常中选择了主要参与者:胰岛素和RNF213。但是在确定RNF213在胰岛素调节途径中的作用之前,我们必须了解RNF213在不同生物系统中的参与。为此,我们采用了一种初步的计算方法来查找RNF213的突出相互作用。我们的第一个目标是为RNF213构建一个相互作用组。我们已经分析了多个精选数据库,并调整了RNF213相互作用伙伴的列表以开发其相互作用组。然后,为了了解此交互组在生物学功能中的参与,我们通过计算方法分析了与该交互组相关的主要生物学途径,生物学过程和重要簇。然后,为了开发一条可能为RNF213参与胰岛素调节途径提供线索的途径,我们验证了簇间和簇内的预测,并确定了RNF213的调节途径。观察到RNF213相互作用组与4个主要簇有关的适应性免疫。其中之一涉及TNFα。免疫系统涉及多种途径,因此,在这一点上,我们选择了基于事件的策略来获得明确的靶标。免疫是由促炎性细胞因子(如TNFα)介导的。TNFα介导的炎症,肥胖和胰岛素抵抗相关。因此,我们选择探索RNF213在巨噬细胞中TNFα介导的炎症和在脂肪细胞中炎症介导的胰岛素抵抗中的作用。我们已经观察到LPS介导的促炎性刺激增强了RNF213基因的表达,并在巨噬细胞以及脂肪细胞中受到了PPARγ介导的抗炎性,胰岛素敏感性的刺激的抑制。促炎细胞因子TNFα的使用能够阻止脂肪形成过程中RNF213表达的降低,并且观察到这种作用是由PTP1B介导的。PTP1B的失活消除了RNF213的表达,从而通过增强的PPARγ增强了脂肪形成过程。RNF213的组成型表达通过抑制PPARγ抑制脂肪细胞分化。我们可以显示出TNFα/ PTP1B途径和PPARγ对RNF213的调节。RNF213在脂肪形成过程中的组成性表达似乎是肥胖患者获得的抑制进一步脂肪形成的脂肪抑制措施。这已验证通过分析从Gene Expression Omnibus数据库获得的基因表达数据,在计算机上进行了研究,结果显示RNF213在肥胖人群的脂肪组织样本中的表达较高。总体而言,这项研究为TNFα介导的脂肪形成途径提供了新的见解,并提出了RNF213在脂肪形成过程中的作用。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
New insights into TNFα/PTP1B and PPARγ pathway through RNF213- a link between inflammation, obesity, insulin resistance, and Moyamoya disease
Diabetic patients are always at a higher risk of ischemic diseases like coronary artery diseases. One such ischemic carotid artery disease is Moyamoya disease (MMD) associated with diabetes Type I and II, but the causality was unclear. Ring Finger Protein 213 (RNF213) is the major susceptible gene for MMD. To understand the association between diabetes mellitus and MMD we chose the major players from both of the anomalies: insulin and RNF213. But before establishing the role of RNF213 in the insulin-regulating pathway we had to understand the involvement of RNF213 within different biological systems. For this, we have adopted a preliminary computational approach to find the prominent interactions of RNF213. Our first objective was to construct an interactome for RNF213. We have analyzed several curated databases and adapted a list of RNF213 interacting partners to develop its interactome. Then to understand the involvement of this interactome in biological functions we have analyzed major biological pathways, biological processes, and prominent clusters related to this interactome through a computational approach. Then to develop a pathway that might give clues for RNF213 involvement in the insulin regulatory pathway we have validated the intercluster and intracluster predictions and identified a regulatory pathway for RNF213. RNF213 interactome was observed to be involved in adaptive immunity with 4 major clusters; one of the clusters involved TNFα. The immune system involves several pathways, and therefore at this point, we have chosen an event-based strategy to obtain an explicit target. Immunity is mediated by pro-inflammatory cytokines like TNFα. TNFα-mediated inflammation, obesity, and insulin resistance are associated. Therefore we chose to explore the role of RNF213 in TNFα-mediated inflammation in macrophages and inflammation-mediated insulin-resistance in adipocytes. We have observed an enhancement of RNF213 gene expression by LPS mediated pro-inflammatory stimuli and suppression by PPARγ-mediated anti-inflammatory, insulin-sensitizing stimuli in macrophages, and also in adipocytes. Administration of the pro-inflammatory cytokine TNFα was able to impede the reduction in RNF213 expression during adipogenesis and this effect was observed to be mediated by PTP1B. Inactivation of PTP1B abolished RNF213 expression which in turn enhanced the adipogenesis process through enhanced PPARγ. Constitutive expression of RNF213 suppressed the adipocyte differentiation by the inhibition of PPARγ. We could show the regulation of RNF213 by TNFα/PTP1B pathway and PPARγ. The constitutive expression of RNF213 during adipogenesis appears to be an adipostatic measure that obese patients acquire to inhibit further adipogenesis. This is verified in silico by analyzing the gene expression data obtained from the Gene Expression Omnibus database, which showed a higher expression of RNF213 in adipose tissue samples of obese people. Overall this study gives new insights into the TNFα-mediated pathway in adipogenesis and suggests the role of RNF213 in adipogenesis via this pathway.


































 京公网安备 11010802027423号
京公网安备 11010802027423号