当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenanthrene, 9,10-dihydrophenanthrene and bibenzyl enantiomers from Bletilla striata with their antineuroinflammatory and cytotoxic activities
Phytochemistry ( IF 3.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.phytochem.2020.112609 Mo-Han Sun , Xian-Jie Ma , Si-Yuan Shao , Shao-Wei Han , Jian-Wei Jiang , Jian-Jun Zhang , Shuai Li
Phytochemistry ( IF 3.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.phytochem.2020.112609 Mo-Han Sun , Xian-Jie Ma , Si-Yuan Shao , Shao-Wei Han , Jian-Wei Jiang , Jian-Jun Zhang , Shuai Li
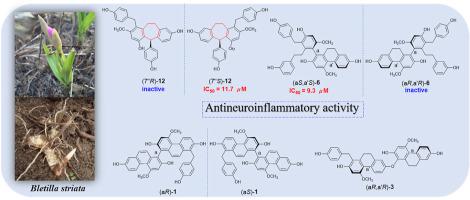
|
Thirteen undescribed phenanthrene and bibenzyl derivatives, named blestanols A-M, including one pair of biphenanthrene enantiomers, two bis 9,10-dihydrophenanthrene ethers, five pairs of 9,10-dihydrophenanthrene/bibenzyl atropisomers, one racemic 9,10-dihydrophenanthrene/bibenzyl dimer, one 9,10-dihydrophenanthrenebibenzyl ether, two pairs of bibenzyl derivatives, and one stilbene, together with 12 known analogues were isolated from the tubers of Bletilla striata. The structures were elucidated via spectroscopic data analysis. 15 compounds were purified to yield enantiomers (a, b) via chiral-phase HPLC, and their configurations were determined by optical rotation values and the comparison of the experimental and calculated electronic circular dichroism (ECD) curves. Blestanols K-L possessed a cycloheptene moiety, which is rarely observed in bibenzyl derivatives. A putative biosynthetic pathway for the identified components is deduced. Among these compounds, 14 compounds showed inhibition of NO production, with IC50 values ranging from 5.0 to 19.0 μM. Eight compounds displayed selective cytotoxic activities against HCT-116, HepG2, BGC-823, A549 or U251 cancer cell lines, with IC50 values ranging from 1.4 to 8.3 μM. In addition, their structure-activity relationships are discussed briefly.
中文翻译:

来自白芨的菲、9,10-二氢菲和联苄基对映异构体及其抗神经炎症和细胞毒活性
13 种未描述的菲和联苄基衍生物,命名为 blestanols AM,包括一对联菲对映异构体、两种双 9,10-二氢菲醚、五对 9,10-二氢菲/联苄基阻转异构体、一种外消旋的 9,10-二氢苯并菲从 Bletilla striata 的块茎中分离出一种 9,10-二氢菲联苄基醚、两对联苄基衍生物和一种芪,以及 12 种已知的类似物。通过光谱数据分析阐明了结构。通过手性相 HPLC 纯化 15 种化合物以产生对映异构体 (a, b),并通过旋光度值以及实验和计算电子圆二色性 (ECD) 曲线的比较确定它们的构型。Blestanols KL 具有环庚烯部分,这在联苄基衍生物中很少观察到。推断出已鉴定成分的推定生物合成途径。在这些化合物中,14 种化合物显示出抑制 NO 产生,IC50 值范围为 5.0 至 19.0 μM。八种化合物对 HCT-116、HepG2、BGC-823、A549 或 U251 癌细胞系表现出选择性细胞毒活性,IC50 值范围为 1.4 至 8.3 μM。此外,简要讨论了它们的构效关系。
更新日期:2021-02-01
中文翻译:

来自白芨的菲、9,10-二氢菲和联苄基对映异构体及其抗神经炎症和细胞毒活性
13 种未描述的菲和联苄基衍生物,命名为 blestanols AM,包括一对联菲对映异构体、两种双 9,10-二氢菲醚、五对 9,10-二氢菲/联苄基阻转异构体、一种外消旋的 9,10-二氢苯并菲从 Bletilla striata 的块茎中分离出一种 9,10-二氢菲联苄基醚、两对联苄基衍生物和一种芪,以及 12 种已知的类似物。通过光谱数据分析阐明了结构。通过手性相 HPLC 纯化 15 种化合物以产生对映异构体 (a, b),并通过旋光度值以及实验和计算电子圆二色性 (ECD) 曲线的比较确定它们的构型。Blestanols KL 具有环庚烯部分,这在联苄基衍生物中很少观察到。推断出已鉴定成分的推定生物合成途径。在这些化合物中,14 种化合物显示出抑制 NO 产生,IC50 值范围为 5.0 至 19.0 μM。八种化合物对 HCT-116、HepG2、BGC-823、A549 或 U251 癌细胞系表现出选择性细胞毒活性,IC50 值范围为 1.4 至 8.3 μM。此外,简要讨论了它们的构效关系。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号