当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemistry of Defects in Crystalline Na2Se: Implications for the Na–Se Battery
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.jpcc.0c09021 Zhixiao Liu 1 , Wangyu Hu 1 , Huiqiu Deng 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.jpcc.0c09021 Zhixiao Liu 1 , Wangyu Hu 1 , Huiqiu Deng 2
Affiliation
|
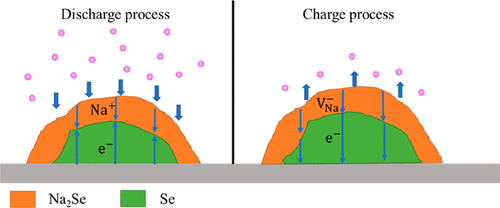
Selenium (Se) is a promising cathode material for next-generation alkali metal–ion batteries due to its high volumetric capacity and good conductivity. Using a first-principles approach, the present study systematically studies the electronic structure and the chemistry of defects of the final discharge product Na2Se for Na–Se batteries. It is found that Na2Se is insulating for free electron transfer. The charged native defects can act as carriers to transfer charges through crystalline Na2Se. The hole polaron p+ has a very low diffusion barrier of 76 meV. However, p+ makes a minor contribution to the conductivity of Na2Se due to its high formation energy of 1.92 eV. In the Na2Se crystal, the negatively charged Na vacancy (VNa–) is the dominant defect with a formation energy of 0.71 eV, and its diffusion barrier is 258 meV which is much lower than that for other vacancies and interstitial defects. According to the present theoretical study, VNa– is the main charge carrier, and the corresponding ionic conductivity is 10–13 S cm–1 at the room temperature. Such a low conductivity can limit the utilization of the active material and reduce the discharge voltage of Na–Se batteries, especially for Na–Se batteries based on the solid–solid reaction mechanism.
中文翻译:

Na 2 Se结晶缺陷的化学性质:对Na-Se电池的影响
硒(Se)由于其高容量和良好的导电性而成为下一代碱金属离子电池的有希望的正极材料。使用第一原理的方法,本研究系统地研究了Na-Se电池最终放电产物Na 2 Se的电子结构和缺陷的化学性质。发现Na 2 Se对于自由电子转移是绝缘的。带电的天然缺陷可以充当载流子,以通过晶体Na 2 Se转移电荷。空穴极化子p +具有非常低的76 meV的扩散势垒。但是,p +对Na 2的电导率贡献很小。硒由于其1.92 eV的高形成能。以Na 2硒晶体,带负电荷的Na空位(V钠- )是主要的缺陷与0.71 2eV的形成能,并且其扩散阻挡是258兆电子伏,其多比用于其它空位和填隙原子缺陷降低。根据目前的理论研究,V Na –是主要的电荷载体,在室温下相应的离子电导率为10 –13 S cm –1。如此低的电导率会限制活性物质的利用并降低Na-Se电池的放电电压,尤其是基于固-固反应机理的Na-Se电池。
更新日期:2020-12-24
中文翻译:

Na 2 Se结晶缺陷的化学性质:对Na-Se电池的影响
硒(Se)由于其高容量和良好的导电性而成为下一代碱金属离子电池的有希望的正极材料。使用第一原理的方法,本研究系统地研究了Na-Se电池最终放电产物Na 2 Se的电子结构和缺陷的化学性质。发现Na 2 Se对于自由电子转移是绝缘的。带电的天然缺陷可以充当载流子,以通过晶体Na 2 Se转移电荷。空穴极化子p +具有非常低的76 meV的扩散势垒。但是,p +对Na 2的电导率贡献很小。硒由于其1.92 eV的高形成能。以Na 2硒晶体,带负电荷的Na空位(V钠- )是主要的缺陷与0.71 2eV的形成能,并且其扩散阻挡是258兆电子伏,其多比用于其它空位和填隙原子缺陷降低。根据目前的理论研究,V Na –是主要的电荷载体,在室温下相应的离子电导率为10 –13 S cm –1。如此低的电导率会限制活性物质的利用并降低Na-Se电池的放电电压,尤其是基于固-固反应机理的Na-Se电池。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号