当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed C–H/N–H Alkyne Annulation of Nonsymmetric 2-Aryl (Benz)imidazole Derivatives: Photophysical and Mechanistic Insights
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.joc.0c02054 Gleiston G. Dias 1 , Esther R. S. Paz 1 , Juliana Y. Kadooca 1 , Adão A. Sabino 1 , Luiz A. Cury 2 , Kohei Torikai 3 , Carlos A. de Simone 4 , Felipe Fantuzzi 5 , Eufrânio N. da Silva Júnior 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.joc.0c02054 Gleiston G. Dias 1 , Esther R. S. Paz 1 , Juliana Y. Kadooca 1 , Adão A. Sabino 1 , Luiz A. Cury 2 , Kohei Torikai 3 , Carlos A. de Simone 4 , Felipe Fantuzzi 5 , Eufrânio N. da Silva Júnior 1
Affiliation
|
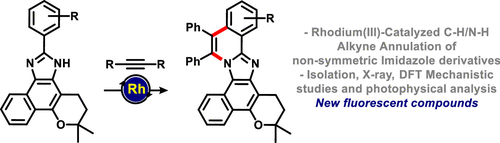
Rhodium(III) catalysis enabled C–H/N–H alkyne annulation of nonsymmetric imidazole derivatives. This study encompasses the synthesis of imidazoles from a naturally occurring quinoidal compound and their use for the preparation of rigid π-extended imidazole derivatives with outstanding fluorescence. Our study also brings to light the photophysical aspects and the mechanism of the reaction studied via computational calculations. This method provided an efficient and versatile tool for the synthesis of fluorescent compounds with a wide range of chemical and biological applications.
中文翻译:

铑(III)催化的非对称2-芳基(Benz)咪唑衍生物的CH / NH炔烃环化:光物理和机械学的见解
铑(III)催化可实现非对称咪唑衍生物的C–H / N–H炔烃环化。这项研究涵盖了由天然存在的醌型化合物合成咪唑及其在制备具有出色荧光性的刚性π-延伸咪唑衍生物中的用途。我们的研究还揭示了光物理方面以及通过计算计算研究的反应机理。该方法为合成具有多种化学和生物学应用的荧光化合物提供了一种有效且通用的工具。
更新日期:2021-01-01
中文翻译:

铑(III)催化的非对称2-芳基(Benz)咪唑衍生物的CH / NH炔烃环化:光物理和机械学的见解
铑(III)催化可实现非对称咪唑衍生物的C–H / N–H炔烃环化。这项研究涵盖了由天然存在的醌型化合物合成咪唑及其在制备具有出色荧光性的刚性π-延伸咪唑衍生物中的用途。我们的研究还揭示了光物理方面以及通过计算计算研究的反应机理。该方法为合成具有多种化学和生物学应用的荧光化合物提供了一种有效且通用的工具。






























 京公网安备 11010802027423号
京公网安备 11010802027423号