当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkaline Phosphatases: in Silico Study on the Catalytic Effect of Conserved Active Site Residues Using Human Placental Alkaline Phosphatase (PLAP) As a Model Protein
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.jcim.0c00860
Gabriela L Borosky 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.jcim.0c00860
Gabriela L Borosky 1
Affiliation
|
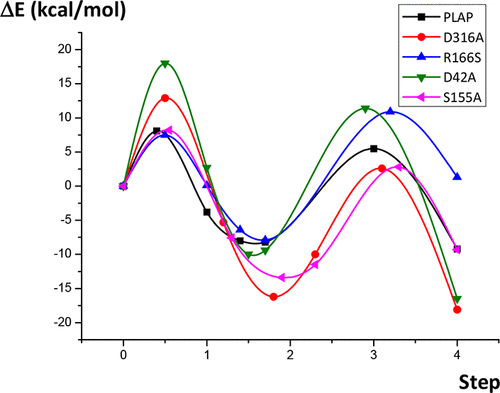
The metalloenzymes from the alkaline phosphatase (AP) superfamily catalyze the hydrolysis and transphosphorylation of phosphate monoesters. The role of several amino acids highly conserved in the active site of this family of enzymes was examined, using human placental AP (PLAP) as a model protein. By employing an active-site model based on the X-ray crystal structure of PLAP, mutations of several key residues were modeled by quantum mechanical methods in order to determine their impact on the catalytic activity. Kinetic and thermodynamic estimations were achieved for each reaction step of the catalytic mechanism by characterization of the intermediates and transition states on the reaction pathway, and the effects of mutations on the activation barriers were analyzed. A good accordance was observed between the present computational results and experimental measurements reported in the literature.
中文翻译:

碱性磷酸酶:使用人胎盘碱性磷酸酶(PLAP)作为模型蛋白的保守活性位点残基催化作用的计算机模拟研究
来自碱性磷酸酶(AP)超家族的金属酶催化磷酸单酯的水解和转磷酸化。使用人胎盘AP(PLAP)作为模型蛋白,检查了在该酶家族的活性位点中高度保守的几种氨基酸的作用。通过使用基于PLAP的X射线晶体结构的活性位点模型,通过量子力学方法对几个关键残基的突变进行了建模,以确定它们对催化活性的影响。通过表征反应路径上的中间体和过渡态,对催化机理的每个反应步骤进行了动力学和热力学估算,并分析了突变对活化障碍的影响。
更新日期:2020-12-28
中文翻译:

碱性磷酸酶:使用人胎盘碱性磷酸酶(PLAP)作为模型蛋白的保守活性位点残基催化作用的计算机模拟研究
来自碱性磷酸酶(AP)超家族的金属酶催化磷酸单酯的水解和转磷酸化。使用人胎盘AP(PLAP)作为模型蛋白,检查了在该酶家族的活性位点中高度保守的几种氨基酸的作用。通过使用基于PLAP的X射线晶体结构的活性位点模型,通过量子力学方法对几个关键残基的突变进行了建模,以确定它们对催化活性的影响。通过表征反应路径上的中间体和过渡态,对催化机理的每个反应步骤进行了动力学和热力学估算,并分析了突变对活化障碍的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号