当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Herbicidal Activity of N-Benzyl-5-cyclopropyl-isoxazole-4-carboxamides
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.jafc.0c03582 Xin-Lin Sun 1 , Zhen-Meng Ji 1 , Shao-Peng Wei 1, 2 , Zhi-Qin Ji 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.jafc.0c03582 Xin-Lin Sun 1 , Zhen-Meng Ji 1 , Shao-Peng Wei 1, 2 , Zhi-Qin Ji 1, 2
Affiliation
|
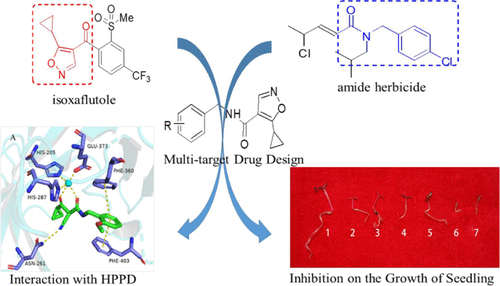
Based on the structures of isoxaflutole (IFT) and N-isobutyl-N-(4-chloro-benzyl)-4-chloro-2-pentenamide, a series of N-benzyl-5-cyclopropyl-isoxazole-4-carboxamides was designed by connecting their pharmacophores (i.e., a multitarget drug design strategy). A total of 27 N-benzyl-5-cyclopropyl-isoxazole-4-carboxamides were prepared from 5-cyclopropylisoxazole-4-carboxylic acid and substituted benzylamines, and their structures were confirmed by NMR and MS. Laboratory bioassays indicated that I-26 showed 100% inhibition against Portulaca oleracea and Abutilon theophrasti at a concentration of 10 mg/L, better than the positive control butachlor (50% inhibition for both weeds). A strong growth inhibition was observed, but a typical bleaching phenomenon of IFT could not be observed in the Petri dish assay. I-05 displayed excellent postemergence herbicidal activity against Echinochloa crusgalli and A. theophrasti at a rate of 150 g/ha, and bleaching symptoms were observed in the leaves of treated weeds. The bleaching effect of Chlamydomonas reinhardtii treated by I-05 could be reversed by adding homogentisate. Enzymatic bioassays indicated that I-05 could not inhibit 4-hydroxyphenylpyruvate dioxygenase (HPPD) activity, but II-05, an isoxazole ring-opening product of I-05, could inhibit HPPD activity with an EC50 value of 1.05 μM, similar to that of mesotrione (with an EC50 value of 1.35 μM). Detailed discussion about observed herbicidal symptoms is provided in the Results and Discussion section. This investigation provided a proof-of-concept foundation that a multitarget drug design strategy could be applied in agrochemical research.
中文翻译:

N-苄基-5-环丙基-异恶唑-4-羧酰胺的设计,合成及除草活性
基于异氟丁环(IFT)和N-异丁基-N-(4-氯苄基)-4-氯-2-戊酰胺的结构,设计了一系列N-苄基-5-环丙基-异恶唑-4-羧酰胺通过连接其药效团(即多目标药物设计策略)。由5-环丙基异恶唑-4-羧酸和取代的苄胺总共制备了27种N-苄基-5-环丙基-异恶唑-4-羧酰胺,其结构通过NMR和MS证实。实验室生物测定表明,I-26显示出对100%抑制马齿苋和苘麻浓度为10 mg / L,优于阳性对照丁草胺(两种杂草均抑制50%)。观察到强烈的生长抑制,但是在培养皿测定中未观察到典型的IFT漂白现象。I-05以150 g / ha的速率显示出优异的除草cru和嗜碱芽孢杆菌的除草活性,并且在处理过的杂草叶片中观察到漂白症状。I-05处理的莱茵衣藻的漂白作用可以通过添加尿黑素来逆转。酶促生物测定表明,I-05不能抑制4-羟苯基丙酮酸双加氧酶(HPPD)的活性,而II-05I-05的异恶唑开环产物可以抑制HPPD活性,其EC 50值为1.05μM,与甲基磺草酮相似(EC 50值为1.35μM)。结果和讨论部分提供了有关所观察到的除草症状的详细讨论。这项研究提供了概念证明的基础,即多目标药物设计策略可用于农业化学研究。
更新日期:2020-12-23
中文翻译:

N-苄基-5-环丙基-异恶唑-4-羧酰胺的设计,合成及除草活性
基于异氟丁环(IFT)和N-异丁基-N-(4-氯苄基)-4-氯-2-戊酰胺的结构,设计了一系列N-苄基-5-环丙基-异恶唑-4-羧酰胺通过连接其药效团(即多目标药物设计策略)。由5-环丙基异恶唑-4-羧酸和取代的苄胺总共制备了27种N-苄基-5-环丙基-异恶唑-4-羧酰胺,其结构通过NMR和MS证实。实验室生物测定表明,I-26显示出对100%抑制马齿苋和苘麻浓度为10 mg / L,优于阳性对照丁草胺(两种杂草均抑制50%)。观察到强烈的生长抑制,但是在培养皿测定中未观察到典型的IFT漂白现象。I-05以150 g / ha的速率显示出优异的除草cru和嗜碱芽孢杆菌的除草活性,并且在处理过的杂草叶片中观察到漂白症状。I-05处理的莱茵衣藻的漂白作用可以通过添加尿黑素来逆转。酶促生物测定表明,I-05不能抑制4-羟苯基丙酮酸双加氧酶(HPPD)的活性,而II-05I-05的异恶唑开环产物可以抑制HPPD活性,其EC 50值为1.05μM,与甲基磺草酮相似(EC 50值为1.35μM)。结果和讨论部分提供了有关所观察到的除草症状的详细讨论。这项研究提供了概念证明的基础,即多目标药物设计策略可用于农业化学研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号