当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Benzothiophene‐Fused Polycyclic, Eight‐Membered N‐Heterocycles via Amine‐Mediated Three‐Component Domino Strategy
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-11 , DOI: 10.1002/adsc.202001129 Qingsong Deng 1 , Aimin Yu 1 , Lei Zhang 2, 3 , Xiangtai Meng 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-11 , DOI: 10.1002/adsc.202001129 Qingsong Deng 1 , Aimin Yu 1 , Lei Zhang 2, 3 , Xiangtai Meng 1
Affiliation
|
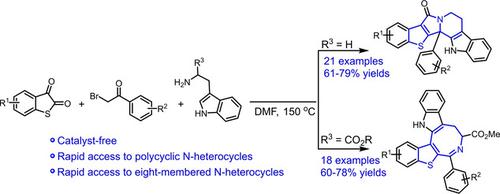
A product‐selective strategy was used to synthesize benzothiophene‐fused polycyclic, eight‐membered N‐heterocycles via a three‐component domino reaction of thioisatins under catalyst‐free conditions. The reaction between tryptamine, thioisatin, and bromoacetophenone produced benzothiophene‐fused polycyclic compounds. In contrast, using D‐tryptophan methyl ester hydrochloride instead of tryptamine afforded benzothiophene‐fused eight‐membered N‐heterocycles. DFT calculations showed that the benzothiophene‐fused polycyclic compounds formed via the Pictet‐Spengler reaction. However, the ester group in D‐tryptophan methyl ester hydrochloride changed the reaction pathway and produced benzothiophene‐fused eight‐membered N‐heterocycles.
中文翻译:

通过胺介导的三组分多米诺骨牌战略选择性合成苯并噻吩融合的多环,八元N-杂环
产品选择策略用于在无催化剂条件下通过硫代靛红的三组分多米诺反应合成苯并噻吩稠合的多环,八元N-杂环。色胺,硫代抑素和溴苯乙酮之间的反应产生了苯并噻吩稠合的多环化合物。相比之下,使用D-色氨酸甲酯盐酸盐代替色胺可得到苯并噻吩稠合的八元N-杂环。DFT计算表明,通过Pictet-Spengler反应形成了苯并噻吩稠合的多环化合物。然而,D-色氨酸甲酯盐酸盐中的酯基改变了反应途径,并生成了苯并噻吩稠合的八元N-杂环。
更新日期:2021-02-16
中文翻译:

通过胺介导的三组分多米诺骨牌战略选择性合成苯并噻吩融合的多环,八元N-杂环
产品选择策略用于在无催化剂条件下通过硫代靛红的三组分多米诺反应合成苯并噻吩稠合的多环,八元N-杂环。色胺,硫代抑素和溴苯乙酮之间的反应产生了苯并噻吩稠合的多环化合物。相比之下,使用D-色氨酸甲酯盐酸盐代替色胺可得到苯并噻吩稠合的八元N-杂环。DFT计算表明,通过Pictet-Spengler反应形成了苯并噻吩稠合的多环化合物。然而,D-色氨酸甲酯盐酸盐中的酯基改变了反应途径,并生成了苯并噻吩稠合的八元N-杂环。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号