当前位置:
X-MOL 学术
›
Food Sci. Nutr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tyrosinase inhibition by p‐coumaric acid ethyl ester identified from camellia pollen
Food Science & Nutrition ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/fsn3.2004
Lijun Li 1, 2, 3 , Yuchen Cai 1 , Xu Sun 1 , Xiping Du 1, 2, 3 , Zedong Jiang 1, 2, 3 , Hui Ni 1, 2, 3 , Yuanfan Yang 1, 2, 3 , Feng Chen 1, 4
Food Science & Nutrition ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/fsn3.2004
Lijun Li 1, 2, 3 , Yuchen Cai 1 , Xu Sun 1 , Xiping Du 1, 2, 3 , Zedong Jiang 1, 2, 3 , Hui Ni 1, 2, 3 , Yuanfan Yang 1, 2, 3 , Feng Chen 1, 4
Affiliation
|
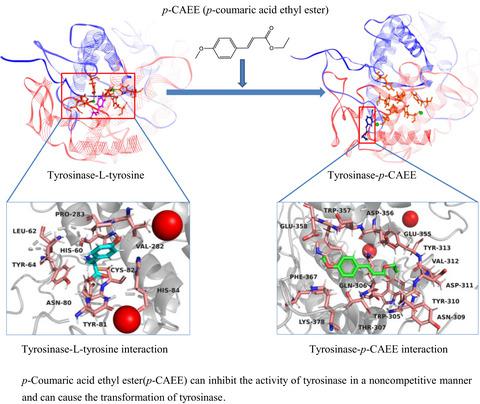
A tyrosinase inhibitor was separated from camellia pollen with the aid of solvent fraction, macroporous adsorptive resin chromatography, and high‐speed countercurrent chromatography. The inhibitor was identified to be p‐coumaric acid ethyl ester (p‐CAEE) by nuclear magnetic resonance and mass spectrum. Its inhibitory activity (IC50 = 4.89 μg/ml) was about 10‐fold stronger than arbutin (IC50 = 51.54 μg/ml). The p‐CAEE inhibited tyrosinase in a noncompetitive model with the KI and Km of 1.83 μg/ml and 0.52 mM, respectively. Fluorescence spectroscopy analysis showed the p‐CAEE quenched an intrinsic fluorescence tyrosinase. UV‐Vis spectroscopy analysis showed the p‐CAEE did not interact with copper ions of the enzyme. Docking simulation implied the p‐CAEE induced a conformational change in the catalytic region and thus changed binding forces of L‐tyrosine. Our findings suggest that p‐CAEE plays an important role in inhibiting tyrosinase and provides a reference for developing pharmaceutical, cosmetic, and fruit preservation products using pollen.
中文翻译:

从山茶花粉中鉴定出对香豆酸乙酯对酪氨酸酶的抑制作用
采用溶剂馏分法、大孔吸附树脂色谱法和高速逆流色谱法从山茶花粉中分离得到酪氨酸酶抑制剂。经核磁共振和质谱鉴定,该抑制剂为对香豆酸乙酯( p -CAEE)。其抑制活性(IC 50 = 4.89 μg/ml)比熊果苷(IC 50 = 51.54 μg/ml)强约10倍。 p -CAEE 在非竞争性模型中抑制酪氨酸酶, K I和K m分别为 1.83 μg/ml 和 0.52 mM。荧光光谱分析表明p -CAEE 猝灭了内在的荧光酪氨酸酶。紫外-可见光谱分析表明p -CAEE 不与酶的铜离子相互作用。对接模拟表明p- CAEE 诱导催化区域构象变化,从而改变 L-酪氨酸的结合力。我们的研究结果表明p -CAEE在抑制酪氨酸酶方面发挥着重要作用,并为利用花粉开发药物、化妆品和水果保鲜产品提供了参考。
更新日期:2021-01-12
中文翻译:

从山茶花粉中鉴定出对香豆酸乙酯对酪氨酸酶的抑制作用
采用溶剂馏分法、大孔吸附树脂色谱法和高速逆流色谱法从山茶花粉中分离得到酪氨酸酶抑制剂。经核磁共振和质谱鉴定,该抑制剂为对香豆酸乙酯( p -CAEE)。其抑制活性(IC 50 = 4.89 μg/ml)比熊果苷(IC 50 = 51.54 μg/ml)强约10倍。 p -CAEE 在非竞争性模型中抑制酪氨酸酶, K I和K m分别为 1.83 μg/ml 和 0.52 mM。荧光光谱分析表明p -CAEE 猝灭了内在的荧光酪氨酸酶。紫外-可见光谱分析表明p -CAEE 不与酶的铜离子相互作用。对接模拟表明p- CAEE 诱导催化区域构象变化,从而改变 L-酪氨酸的结合力。我们的研究结果表明p -CAEE在抑制酪氨酸酶方面发挥着重要作用,并为利用花粉开发药物、化妆品和水果保鲜产品提供了参考。

































 京公网安备 11010802027423号
京公网安备 11010802027423号