当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activatable Polymer Nanoenzymes for Photodynamic Immunometabolic Cancer Therapy
Advanced Materials ( IF 27.4 ) Pub Date : 2020-12-11 , DOI: 10.1002/adma.202007247 Ziling Zeng 1 , Chi Zhang 1 , Jingchao Li 1 , Dong Cui 1 , Yuyan Jiang 1 , Kanyi Pu 1
Advanced Materials ( IF 27.4 ) Pub Date : 2020-12-11 , DOI: 10.1002/adma.202007247 Ziling Zeng 1 , Chi Zhang 1 , Jingchao Li 1 , Dong Cui 1 , Yuyan Jiang 1 , Kanyi Pu 1
Affiliation
|
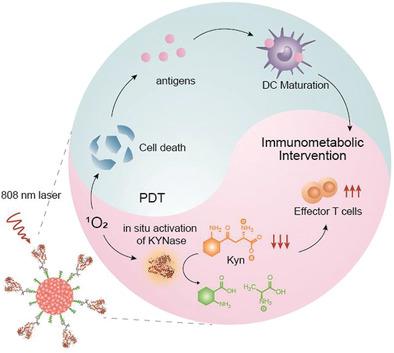
Tumor immunometabolism contributes substantially to tumor proliferation and immune cell activity, and thus plays a crucial role in the efficacy of cancer immunotherapy. Modulation of immunometabolism to boost cancer immunotherapy is mostly based on small‐molecule inhibitors, which often encounter the issues of off‐target adverse effects, drug resistance, and unsustainable response. In contrast, enzymatic therapeutics can potentially bypass these limitations but has been less exploited. Herein, an organic polymer nanoenzyme (SPNK) with near‐infrared (NIR) photoactivatable immunotherapeutic effects is reported for photodynamic immunometabolic therapy. SPNK is composed of a semiconducting polymer core conjugated with kynureninase (KYNase) via PEGylated singlet oxygen (1O2) cleavable linker. Upon NIR photoirradiation, SPNK generates 1O2 not only to exert photodynamic effect to induce the immunogenic cell death of cancer, but also to unleash KYNase and trigger its activity to degrade the immunosuppressive kynurenine (Kyn). Such a combinational effect mediated by SPNK promotes the proliferation and infiltration of effector T cells, enhances systemic antitumor T cell immunity, and ultimately permits inhibition of both primary and distant tumors in living mice. Therefore, this study provides a promising photodynamic approach toward remotely controlled enzymatic immunomodulation for improved anticancer therapy.
中文翻译:

用于光动力免疫代谢癌症治疗的可激活聚合物纳米酶
肿瘤免疫代谢对肿瘤增殖和免疫细胞活性有很大贡献,因此对癌症免疫治疗的疗效起着至关重要的作用。调节免疫代谢来促进癌症免疫治疗大多基于小分子抑制剂,经常遇到脱靶不良反应、耐药性和不可持续反应等问题。相比之下,酶疗法有可能绕过这些限制,但较少被利用。在此,报道了一种具有近红外(NIR)光激活免疫治疗作用的有机聚合物纳米酶(SPNK)用于光动力免疫代谢疗法。 SPNK 由通过聚乙二醇化单线态氧 ( 1 O 2 ) 可裂解接头与犬尿氨酸酶 (KYNase) 缀合的半导体聚合物核心组成。在近红外光照射下,SPNK 产生1 O 2 ,不仅发挥光动力效应诱导癌症的免疫原性细胞死亡,而且还释放 KYNase 并触发其活性,降解免疫抑制性犬尿氨酸 (Kyn)。 SPNK介导的这种组合效应促进效应T细胞的增殖和浸润,增强全身抗肿瘤T细胞免疫,并最终抑制活体小鼠的原发性和远处肿瘤。因此,这项研究为远程控制酶免疫调节提供了一种有前途的光动力方法,以改善抗癌治疗。
更新日期:2021-01-25
中文翻译:

用于光动力免疫代谢癌症治疗的可激活聚合物纳米酶
肿瘤免疫代谢对肿瘤增殖和免疫细胞活性有很大贡献,因此对癌症免疫治疗的疗效起着至关重要的作用。调节免疫代谢来促进癌症免疫治疗大多基于小分子抑制剂,经常遇到脱靶不良反应、耐药性和不可持续反应等问题。相比之下,酶疗法有可能绕过这些限制,但较少被利用。在此,报道了一种具有近红外(NIR)光激活免疫治疗作用的有机聚合物纳米酶(SPNK)用于光动力免疫代谢疗法。 SPNK 由通过聚乙二醇化单线态氧 ( 1 O 2 ) 可裂解接头与犬尿氨酸酶 (KYNase) 缀合的半导体聚合物核心组成。在近红外光照射下,SPNK 产生1 O 2 ,不仅发挥光动力效应诱导癌症的免疫原性细胞死亡,而且还释放 KYNase 并触发其活性,降解免疫抑制性犬尿氨酸 (Kyn)。 SPNK介导的这种组合效应促进效应T细胞的增殖和浸润,增强全身抗肿瘤T细胞免疫,并最终抑制活体小鼠的原发性和远处肿瘤。因此,这项研究为远程控制酶免疫调节提供了一种有前途的光动力方法,以改善抗癌治疗。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号