当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrogenerated Chemiluminescence of Luminol Mediated by Carbonate Electrochemical Oxidation at a Boron-Doped Diamond
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.analchem.0c04212 Irkham 1 , Raishaqy R. Rais 2 , Tribidasari A. Ivandini 2 , Andrea Fiorani 1 , Yasuaki Einaga 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.analchem.0c04212 Irkham 1 , Raishaqy R. Rais 2 , Tribidasari A. Ivandini 2 , Andrea Fiorani 1 , Yasuaki Einaga 1
Affiliation
|
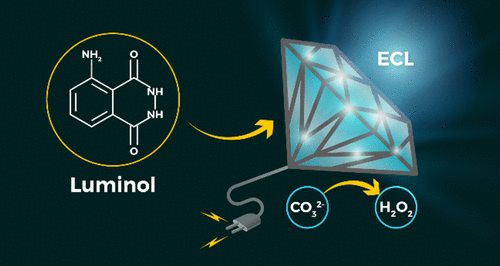
The electrogenerated chemiluminescence of luminol is a process by which light generation is triggered by adding hydrogen peroxide and then applying a suitable electrode potential. Here, we take this phenomenon one step forward by avoiding the addition of hydrogen peroxide using a smart combination of a boron-doped diamond electrode and a carbonate electrolyte to generate the hydrogen peroxide directly in situ. The reaction occurs because of the carbonate electrochemical oxidation to peroxydicarbonate and the following hydrolysis to hydrogen peroxide, which triggers the emission from luminol. The electrogenerated chemiluminescence emission has been optimized by an investigation of the applied potentials, the carbonate concentration, and the pH. Furthermore, these results have been used to shine a light on the reaction mechanisms. Because this method does not require the addition of hydrogen peroxide, it might find application in efforts to avoid instability of hydrogen peroxide or its interference with the analytes of interest.
中文翻译:

掺硼金刚石在碳酸盐电化学氧化作用下鲁米诺的电化学发光
鲁米诺的电化学发光是通过添加过氧化氢然后施加合适的电极电位来触发光产生的过程。在这里,我们通过避免使用掺硼金刚石电极和碳酸盐电解质的聪明组合直接添加过氧化氢来避免过氧化氢,从而使这一现象向前迈进了一步。该反应的发生是由于碳酸盐电化学氧化为过氧化二碳酸盐,然后水解为过氧化氢,这触发了鲁米诺的发射。通过研究所施加的电势,碳酸盐浓度和pH,可以优化电产生的化学发光。此外,这些结果已被用来照亮反应机理。
更新日期:2021-02-02
中文翻译:

掺硼金刚石在碳酸盐电化学氧化作用下鲁米诺的电化学发光
鲁米诺的电化学发光是通过添加过氧化氢然后施加合适的电极电位来触发光产生的过程。在这里,我们通过避免使用掺硼金刚石电极和碳酸盐电解质的聪明组合直接添加过氧化氢来避免过氧化氢,从而使这一现象向前迈进了一步。该反应的发生是由于碳酸盐电化学氧化为过氧化二碳酸盐,然后水解为过氧化氢,这触发了鲁米诺的发射。通过研究所施加的电势,碳酸盐浓度和pH,可以优化电产生的化学发光。此外,这些结果已被用来照亮反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号