当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of a Series of Oxazolone Carboxamides as a Novel Class of Acid Ceramidase Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.jmedchem.0c01561 Samantha Caputo 1, 2 , Simona Di Martino 1, 2 , Vincenzo Cilibrasi 1, 2 , Piero Tardia 1, 2 , Marco Mazzonna 1, 2 , Debora Russo 1, 3 , Ilaria Penna 1, 3 , Maria Summa 1, 4 , Sine Mandrup Bertozzi 1, 4 , Natalia Realini 1, 2 , Natasha Margaroli 1, 2 , Marco Migliore 1, 2 , Giuliana Ottonello 1, 4 , Min Liu 5 , Peter Lansbury 5 , Andrea Armirotti 1, 4 , Rosalia Bertorelli 1, 4 , Soumya S. Ray 5 , Renato Skerlj 5 , Rita Scarpelli 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.jmedchem.0c01561 Samantha Caputo 1, 2 , Simona Di Martino 1, 2 , Vincenzo Cilibrasi 1, 2 , Piero Tardia 1, 2 , Marco Mazzonna 1, 2 , Debora Russo 1, 3 , Ilaria Penna 1, 3 , Maria Summa 1, 4 , Sine Mandrup Bertozzi 1, 4 , Natalia Realini 1, 2 , Natasha Margaroli 1, 2 , Marco Migliore 1, 2 , Giuliana Ottonello 1, 4 , Min Liu 5 , Peter Lansbury 5 , Andrea Armirotti 1, 4 , Rosalia Bertorelli 1, 4 , Soumya S. Ray 5 , Renato Skerlj 5 , Rita Scarpelli 1, 2
Affiliation
|
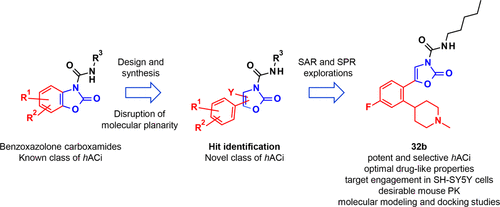
Acid ceramidase (AC) is a cysteine hydrolase that plays a crucial role in the metabolism of lysosomal ceramides, important members of the sphingolipid family, a diversified class of bioactive molecules that mediate many biological processes ranging from cell structural integrity, signaling, and cell proliferation to cell death. In the effort to expand the structural diversity of the existing collection of AC inhibitors, a novel class of substituted oxazol-2-one-3-carboxamides were designed and synthesized. Herein, we present the chemical optimization of our initial hits, 2-oxo-4-phenyl-N-(4-phenylbutyl)oxazole-3-carboxamide 8a and 2-oxo-5-phenyl-N-(4-phenylbutyl)oxazole-3-carboxamide 12a, which resulted in the identification of 5-[4-fluoro-2-(1-methyl-4-piperidyl)phenyl]-2-oxo-N-pentyl-oxazole-3-carboxamide 32b as a potent AC inhibitor with optimal physicochemical and metabolic properties, showing target engagement in human neuroblastoma SH-SY5Y cells and a desirable pharmacokinetic profile in mice, following intravenous and oral administration. 32b enriches the arsenal of promising lead compounds that may therefore act as useful pharmacological tools for investigating the potential therapeutic effects of AC inhibition in relevant sphingolipid-mediated disorders.
中文翻译:

设计,合成和生物评价一系列恶唑酮羧酰胺作为一类新型的酸性神经酰胺酶抑制剂。
酸性神经酰胺酶(AC)是一种半胱氨酸水解酶,在溶酶体神经酰胺,鞘脂家族的重要成员,一种多样化的生物活性分子的代谢中起着至关重要的作用,介导许多生物过程,介导许多生物学过程,包括细胞结构完整性,信号传导和细胞增殖细胞死亡。为了扩大现有AC抑制剂的结构多样性,设计并合成了一类新的取代的恶唑-2-一-3-羧酰胺类。在这里,我们介绍了我们最初的命中2-oxo-4-苯基-N-(4-苯基丁基)恶唑-3-羧酰胺8a和2-oxo-5-苯基-N-(4-苯基丁基)恶唑的化学优化-3-羧酰胺12a,从而鉴定出5- [4-氟-2-(1-甲基-4-哌啶基)苯基] -2-氧代-N-戊基-恶唑-3-羧酰胺32b为具有最佳物理化学作用的强力AC抑制剂以及代谢特性,在静脉和口服给药后,显示了靶标在人神经母细胞瘤SH-SY5Y细胞中的参与以及在小鼠中理想的药代动力学特征。32b丰富了有前途的先导化合物的库,因此可作为有用的药理工具,用于研究在相关鞘脂介导的疾病中AC抑制的潜在治疗作用。
更新日期:2020-12-24
中文翻译:

设计,合成和生物评价一系列恶唑酮羧酰胺作为一类新型的酸性神经酰胺酶抑制剂。
酸性神经酰胺酶(AC)是一种半胱氨酸水解酶,在溶酶体神经酰胺,鞘脂家族的重要成员,一种多样化的生物活性分子的代谢中起着至关重要的作用,介导许多生物过程,介导许多生物学过程,包括细胞结构完整性,信号传导和细胞增殖细胞死亡。为了扩大现有AC抑制剂的结构多样性,设计并合成了一类新的取代的恶唑-2-一-3-羧酰胺类。在这里,我们介绍了我们最初的命中2-oxo-4-苯基-N-(4-苯基丁基)恶唑-3-羧酰胺8a和2-oxo-5-苯基-N-(4-苯基丁基)恶唑的化学优化-3-羧酰胺12a,从而鉴定出5- [4-氟-2-(1-甲基-4-哌啶基)苯基] -2-氧代-N-戊基-恶唑-3-羧酰胺32b为具有最佳物理化学作用的强力AC抑制剂以及代谢特性,在静脉和口服给药后,显示了靶标在人神经母细胞瘤SH-SY5Y细胞中的参与以及在小鼠中理想的药代动力学特征。32b丰富了有前途的先导化合物的库,因此可作为有用的药理工具,用于研究在相关鞘脂介导的疾病中AC抑制的潜在治疗作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号