当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural, Electrical, and Magnetic Versatility of the Oxalate-Based [CuFe] Compounds Containing 2,2′:6′,2″-Terpyridine: Anion-Directed Synthesis
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.inorgchem.0c02548 Lidija Kanižaj 1 , Dario Barišić 2 , Filip Torić 2, 3 , Damir Pajić 2 , Krešimir Molčanov 4 , Ana Šantić 1 , Ivor Lončarić 5 , Marijana Jurić 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.inorgchem.0c02548 Lidija Kanižaj 1 , Dario Barišić 2 , Filip Torić 2, 3 , Damir Pajić 2 , Krešimir Molčanov 4 , Ana Šantić 1 , Ivor Lončarić 5 , Marijana Jurić 1
Affiliation
|
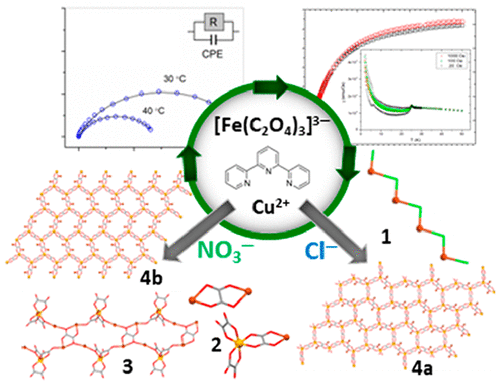
The heterodimetallic [CuFe] compounds [CuII4(terpy)4Cl5][FeIII(C2O4)3]·10H2O (1;terpy = 2,2′:6′,2′′-terpyridine), [CuII2(H2O)2(terpy)2(C2O4)][CuIIFeIII(CH3OH)(terpy)(C2O4)3]2 (2), and {[Cu2IIFeIII(H2O)(terpy)2(C2O4)7/2]·6H2O}n (3) were obtained using building block approach, from reaction of aqueous solution of [Fe(C2O4)3]3– and a methanol solution containing Cu2+ ions and terpy by the layering technique. Interestingly, by changing only the anion of the starting salt of copper(II), Cu(NO3)2·3H2O instead of CuCl2·2H2O, an unexpected change in the type of bridge, oxalate (2 and 3) versus chloride (1), was achieved, thus affecting the overall structural architecture. Two polymorphs of 3D coordination polymer [CuIIFeII2(H2O)(terpy)(C2O4)3]n (4), crystallizing in the triclinic (a) and monoclinic (b) space groups, were formed hydrothermally, depending on whether CuCl2·2H2O or Cu(NO3)2·3H2O was added to the water, besides K3[Fe(C2O4)3]·3H2O and terpy, respectively. Under hydrothermal conditions iron(III) from initial building block is reduced to the divalent state, creating 2D honeycomb [FeII2(C2O4)3]n2n– layers, which are bridged by [Cu(H2O)(terpy)]2+ cations. Compounds were investigated by single-crystal X-ray diffraction, IR, and impedance spectroscopies, magnetization measurements, and density functional theory (DFT) calculations. In compounds 1 and 2, 0D magnetism is observed, with 1 having a ground-state spin of 1 due to different interactions through chloride bridges of Cu2+ ions in tetramer [CuII4(terpy)4Cl5]3+ and 2 showing strong antiferromagnetic coupling of Cu2+ ions mediated by oxalate ligand in [CuII2(H2O)2(terpy)2(C2O4)]2+ and weak ones between Cu2+ and Fe3+ ions through oxalate bridge in [CuIIFeIII(CH3OH)(terpy)(C2O4)3]−. Polymer 4 exhibits antiferromagnetic phase transition at 25 K: The [FeII2(C2O4)3]n2n– layers are antiferromagnetically ordered, and a small amount of interlayer interaction is transferred through [Cu(H2O)(terpy)]2+ cations via Oox–Cu–Oox bridges. Additionally, compounds 1 and 2 are electrical insulators, while 4a and 4b show proton conductivity.
中文翻译:

含有2,2':6',2''-三联吡啶的草酸盐基[CuFe]化合物的结构,电和磁通用性:阴离子定向合成
异二金属[CuFe]化合物[Cu II 4(terpy)4 Cl 5 ] [Fe III(C 2 O 4)3 ]·10H 2 O(1 ; terpy = 2,2':6',2''-吡啶),[Cu II 2(H 2 O)2(叔)2(C 2 O 4)] [Cu II Fe III(CH 3 OH)(叔)(C 2 O 4)3 ] 2(2),和{[Cu 2 IIFe III(H 2 O)(terpy)2(C 2 O 4)7/2 ]·6H 2 O} n(3)是通过[Fe(C 2 O 4))3 ] 3–和通过分层技术形成的含有Cu 2+离子和叔丁基的甲醇溶液。有趣的是,通过仅改变铜(II)的起始盐的阴离子,即可代替CuCl 2 ·2H 2的Cu(NO 3)2 ·3H 2 OO,草酸盐(2和3)对氯化物(1)的类型发生了意想不到的变化,从而影响了整体结构。形成了在三斜晶系(a)和单斜晶系(b)中结晶的3D配位聚合物[Cu II Fe II 2(H 2 O)(terpy)(C 2 O 4)3 ] n(4)的两个多晶型物。水热,取决于CuCl 2 ·2H 2 O或Cu(NO 3)2 ·3H 2除了分别添加K 3 [Fe(C 2 O 4)3 ]·3H 2 O和terpy外,还将O添加到水中。在水热条件下,最初的结构单元中的铁(III)还原为二价态,从而形成二维蜂窝[Fe II 2(C 2 O 4)3 ] n 2 n –层,并由[Cu(H 2 O)桥接(terpy)] 2+阳离子。通过单晶X射线衍射,IR和阻抗谱,磁化强度测量和密度泛函理论(DFT)计算来研究化合物。在化合物1中和2,0D磁性观察,用1具有1基态自旋由于通过铜的氯化物桥不同的相互作用2+离子在四聚体[铜II 4(三联吡啶)4氯5 ] 3+和2示出了反铁磁性强草酸配体介导的Cu 2+离子在[Cu II 2(H 2 O)2(terpy)2(C 2 O 4)] 2+中与弱离子在Cu 2+和Fe 3+之间的偶联离子通过[Cu II Fe III(CH 3 OH)(terpy)(C 2 O 4)3 ] -中的草酸盐桥键。聚合物4在25 K时表现出反铁磁相变:[Fe II 2(C 2 O 4)3 ] n 2 n –层是反铁磁有序的,并且少量的层间相互作用通过[Cu(H 2 O)( [Terpy)]通过O ox –Cu–O ox桥连接2+阳离子。此外,化合物1图1和图2是电绝缘体,而图4a和图4b示出质子传导性。
更新日期:2020-12-21
中文翻译:

含有2,2':6',2''-三联吡啶的草酸盐基[CuFe]化合物的结构,电和磁通用性:阴离子定向合成
异二金属[CuFe]化合物[Cu II 4(terpy)4 Cl 5 ] [Fe III(C 2 O 4)3 ]·10H 2 O(1 ; terpy = 2,2':6',2''-吡啶),[Cu II 2(H 2 O)2(叔)2(C 2 O 4)] [Cu II Fe III(CH 3 OH)(叔)(C 2 O 4)3 ] 2(2),和{[Cu 2 IIFe III(H 2 O)(terpy)2(C 2 O 4)7/2 ]·6H 2 O} n(3)是通过[Fe(C 2 O 4))3 ] 3–和通过分层技术形成的含有Cu 2+离子和叔丁基的甲醇溶液。有趣的是,通过仅改变铜(II)的起始盐的阴离子,即可代替CuCl 2 ·2H 2的Cu(NO 3)2 ·3H 2 OO,草酸盐(2和3)对氯化物(1)的类型发生了意想不到的变化,从而影响了整体结构。形成了在三斜晶系(a)和单斜晶系(b)中结晶的3D配位聚合物[Cu II Fe II 2(H 2 O)(terpy)(C 2 O 4)3 ] n(4)的两个多晶型物。水热,取决于CuCl 2 ·2H 2 O或Cu(NO 3)2 ·3H 2除了分别添加K 3 [Fe(C 2 O 4)3 ]·3H 2 O和terpy外,还将O添加到水中。在水热条件下,最初的结构单元中的铁(III)还原为二价态,从而形成二维蜂窝[Fe II 2(C 2 O 4)3 ] n 2 n –层,并由[Cu(H 2 O)桥接(terpy)] 2+阳离子。通过单晶X射线衍射,IR和阻抗谱,磁化强度测量和密度泛函理论(DFT)计算来研究化合物。在化合物1中和2,0D磁性观察,用1具有1基态自旋由于通过铜的氯化物桥不同的相互作用2+离子在四聚体[铜II 4(三联吡啶)4氯5 ] 3+和2示出了反铁磁性强草酸配体介导的Cu 2+离子在[Cu II 2(H 2 O)2(terpy)2(C 2 O 4)] 2+中与弱离子在Cu 2+和Fe 3+之间的偶联离子通过[Cu II Fe III(CH 3 OH)(terpy)(C 2 O 4)3 ] -中的草酸盐桥键。聚合物4在25 K时表现出反铁磁相变:[Fe II 2(C 2 O 4)3 ] n 2 n –层是反铁磁有序的,并且少量的层间相互作用通过[Cu(H 2 O)( [Terpy)]通过O ox –Cu–O ox桥连接2+阳离子。此外,化合物1图1和图2是电绝缘体,而图4a和图4b示出质子传导性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号